Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial)
- PMID: 35169169
- PMCID: PMC8847351
- DOI: 10.1038/s41598-022-06221-8
Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial)
Abstract
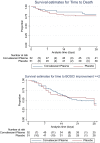
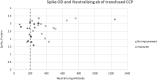
There is a need for effective therapy for COVID-19 pneumonia. Convalescent plasma has antiviral activity and early observational studies suggested benefit in reducing COVID-19 severity. We investigated the safety and efficacy of convalescent plasma in hospitalized patients with COVID-19 in a population with a high HIV prevalence and where few therapeutic options were available. We performed a double-blinded, multicenter, randomized controlled trial in one private and three public sector hospitals in South Africa. Adult participants with COVID-19 pneumonia requiring non-invasive oxygen were randomized 1:1 to receive a single transfusion of 200 mL of either convalescent plasma or 0.9% saline solution. The primary outcome measure was hospital discharge and/or improvement of ≥ 2 points on the World Health Organisation Blueprint Ordinal Scale for Clinical Improvement by day 28 of enrolment. The trial was stopped early for futility by the Data and Safety Monitoring Board. 103 participants, including 21 HIV positive individuals, were randomized at the time of premature trial termination: 52 in the convalescent plasma and 51 in the placebo group. The primary outcome occurred in 31 participants in the convalescent plasma group and and 32 participants in the placebo group (relative risk 1.03 (95% CI 0.77 to 1.38). Two grade 1 transfusion-related adverse events occurred. Participants who improved clinically received convalescent plasma with a higher median anti-SARS-CoV-2 neutralizing antibody titre compared with those who did not (298 versus 205 AU/mL). Our study contributes additional evidence for recommendations against the use of convalescent plasma for COVID-19 pneumonia. Safety and feasibility in this population supports future investigation for other indications.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): A randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- 203135/WT_/Wellcome Trust/United Kingdom
- 203135/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- FC0010218/CRUK_/Cancer Research UK/United Kingdom
- 1D43-TW010345/NIH Fogarty International Center Training Grant
- FC0010218/MRC_/Medical Research Council/United Kingdom
- D43 TW010345/TW/FIC NIH HHS/United States
- K43 TW011421/TW/FIC NIH HHS/United States
- K43TW011421/National Institute of Health, South Africa
- 98341/South African Research Chairs Initiative of the Department of Science and Innovation and the National Research Foundation
- 202254/WT_/Wellcome Trust/United Kingdom
- FC0010218/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

