Development of behavioral patterns in young C57BL/6J mice: a home cage-based study
- PMID: 35169182
- PMCID: PMC8847349
- DOI: 10.1038/s41598-022-06395-1
Development of behavioral patterns in young C57BL/6J mice: a home cage-based study
Abstract
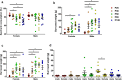
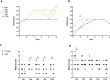
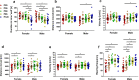
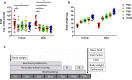
Evidence exists that behavioral patterns only stabilize once mice reach adulthood. Detailed information about the course of behavioral patterns is of particular relevance for neuroscientific research and for the assessment of cumulative severity in genetically modified mice. The analysis considered five age groups focusing on behavioral assessments in the animals' familiar home cage environment during the adolescence phase. We confirmed age- and sex-specific differences for several of the behavioral parameters and fecal corticosterone metabolites. Interestingly, an age-dependent decline in saccharin preference was detected in female mice. Regardless of sex, relevant levels of burrowing activity were only observed during later developmental phases. The development of nest complexity following the offer of new material was affected by age in female mice. In female and male mice, an age-dependency was evident for wheel running reaching a peak at P 50. A progressive increase with age was also observed for Open field activity. The data sets provide guidance for behavioral studies and for development of composite measure schemes for evidence-based severity assessment in young mice. Except for the burrowing test, the different behavioral tests can be applied in different age groups during post-weaning development. However, age- and sex-specific characteristics need to be considered.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

