The role of chromatin loop extrusion in antibody diversification
- PMID: 35169260
- PMCID: PMC9376198
- DOI: 10.1038/s41577-022-00679-3
The role of chromatin loop extrusion in antibody diversification
Abstract
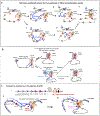
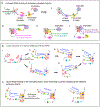
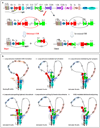
Cohesin mediates chromatin loop formation across the genome by extruding chromatin between convergently oriented CTCF-binding elements. Recent studies indicate that cohesin-mediated loop extrusion in developing B cells presents immunoglobulin heavy chain (Igh) variable (V), diversity (D) and joining (J) gene segments to RAG endonuclease through a process referred to as RAG chromatin scanning. RAG initiates V(D)J recombinational joining of these gene segments to generate the large number of different Igh variable region exons that are required for immune responses to diverse pathogens. Antigen-activated mature B cells also use chromatin loop extrusion to mediate the synapsis, breakage and end joining of switch regions flanking Igh constant region exons during class-switch recombination, which allows for the expression of different antibody constant region isotypes that optimize the functions of antigen-specific antibodies to eliminate pathogens. Here, we review recent advances in our understanding of chromatin loop extrusion during V(D)J recombination and class-switch recombination at the Igh locus.
© 2022. Springer Nature Limited.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

