Skeletal infections: microbial pathogenesis, immunity and clinical management
- PMID: 35169289
- PMCID: PMC8852989
- DOI: 10.1038/s41579-022-00686-0
Skeletal infections: microbial pathogenesis, immunity and clinical management
Abstract
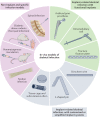
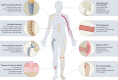
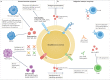
Osteomyelitis remains one of the greatest risks in orthopaedic surgery. Although many organisms are linked to skeletal infections, Staphylococcus aureus remains the most prevalent and devastating causative pathogen. Important discoveries have uncovered novel mechanisms of S. aureus pathogenesis and persistence within bone tissue, including implant-associated biofilms, abscesses and invasion of the osteocyte lacuno-canalicular network. However, little clinical progress has been made in the prevention and eradication of skeletal infection as treatment algorithms and outcomes have only incrementally changed over the past half century. In this Review, we discuss the mechanisms of persistence and immune evasion in S. aureus infection of the skeletal system as well as features of other osteomyelitis-causing pathogens in implant-associated and native bone infections. We also describe how the host fails to eradicate bacterial bone infections, and how this new information may lead to the development of novel interventions. Finally, we discuss the clinical management of skeletal infection, including osteomyelitis classification and strategies to treat skeletal infections with emerging technologies that could translate to the clinic in the future.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

