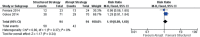Monosymptomatic Nocturnal Enuresis Treatment Using Alarm-Therapy and Desmopressin: A Meta-analysis Approach
- PMID: 35169370
- PMCID: PMC8810148
- DOI: 10.5455/medarh.2021.75.431-435
Monosymptomatic Nocturnal Enuresis Treatment Using Alarm-Therapy and Desmopressin: A Meta-analysis Approach
Abstract
Background: One of the common pediatric issues is monosymptomatic nocturnal enuresis (MNE). MNE is involuntarily urine-voiding in night sleep without lower urinary tract symptoms, such as daytime frequency, incontinence, or urgency. Alarm therapy and desmopressin have been used for treating MNE, but there is no clear comparison of the effectiveness of the two modalities.
Objective: This study aimed to compare the efficacy of alarm therapy and desmopressin and strategies to improve the therapy.
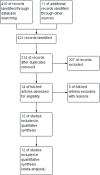
Methods: Study searches were conducted on PubMed, Embase, and Cochrane with a time span of 2010 to 2021. The keywords used were desmopressin, alarm therapy, pediatrics, and monosymptomatic enuresis. The study included an RCT in English, and no subjects were dropped out. Studies without a definite number of subjects were excluded.
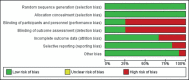
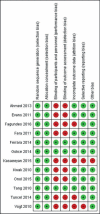
Results: As many as 12 studies were included in the meta-analysis, 9 of which looked for response rates, and 3 were for desmopressin-withdrawal optimization strategy. Alarm therapy was superior to desmopressin in well-motivated parents and patients (p=0.02), with a combined risk ratio of 1.10 in the low heterogeneity population (Z-score = 2.31; I2 = 32%). A strategy that could reduce the risk of desmopressin-withdrawal was a structured dose reduction rather than a sudden dose reduction (p=0.001; I2=0%; Z-score = 3.26). However, therapy discontinuation based on time did not differ the risk (p=0.24; I2=0%; Z-score = 1.17).
Conclusion: The meta-analysis shows that alarm therapy has a better response rate than desmopressin in proactive parents. However, desmopressin may be an option in the opposite subjects, and it is necessary to use structured strategies to optimize the treatment.
Keywords: Alarm therapy; desmopressin; monosymptomatic nocturnal enuresis.
© 2021 Athaya Febriantyo Purnomo, Besut Daryanto, Pradana Nurhadi.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Comparison of desmopressin, alarm, desmopressin plus alarm, and desmopressin plus anticholinergic agents in the management of paediatric monosymptomatic nocturnal enuresis: a network meta-analysis.BJU Int. 2019 Mar;123(3):388-400. doi: 10.1111/bju.14539. Epub 2018 Oct 31. BJU Int. 2019. PMID: 30216627 Review.
-
Comparison of the efficacy of desmopressin fast-melting formulation and enuretic alarm in the treatment of monosymptomatic nocturnal enuresis.J Pediatr Urol. 2020 Oct;16(5):645.e1-645.e7. doi: 10.1016/j.jpurol.2020.07.018. Epub 2020 Jul 26. J Pediatr Urol. 2020. PMID: 32826183
-
Systematic Review and Meta-analysis of Alarm versus Desmopressin Therapy for Pediatric Monosymptomatic Enuresis.Sci Rep. 2018 Nov 13;8(1):16755. doi: 10.1038/s41598-018-34935-1. Sci Rep. 2018. PMID: 30425276 Free PMC article.
-
Evaluation of Urinary Aquaporin 2 and Plasma Copeptin as Biomarkers of Effectiveness of Desmopressin Acetate for the Treatment of Monosymptomatic Nocturnal Enuresis.J Urol. 2017 Oct;198(4):921-927. doi: 10.1016/j.juro.2017.04.088. Epub 2017 Apr 28. J Urol. 2017. PMID: 28457803 Clinical Trial.
-
A prospective and randomized study comparing the use of alarms, desmopressin and imipramine in the treatment of monosymptomatic nocturnal enuresis.J Pediatr Urol. 2023 Jun;19(3):241-246. doi: 10.1016/j.jpurol.2023.01.004. Epub 2023 Jan 11. J Pediatr Urol. 2023. PMID: 36717289 Clinical Trial.
Cited by
-
Methylenetetrahydrofolate Reductase C677T (rs1801133) Polymorphism Is Associated with Bladder Cancer in Asian Population: Epigenetic Meta-Analysis as Precision Medicine Approach.Cancers (Basel). 2023 Sep 2;15(17):4402. doi: 10.3390/cancers15174402. Cancers (Basel). 2023. PMID: 37686678 Free PMC article. Review.
-
Comparison of the Efficacy of Tolterodine versus Oxybutynin in the Treatment of Children with Desmopressin-Resistant Enuresis: A Randomized Controlled Clinical Trial.Ethiop J Health Sci. 2023 Jul;33(4):611-620. doi: 10.4314/ejhs.v33i4.7. Ethiop J Health Sci. 2023. PMID: 38784212 Free PMC article. Clinical Trial.
-
Modeling the functionalized genistein-hyoscyamine derivatives.Sci Rep. 2025 May 13;15(1):16662. doi: 10.1038/s41598-025-00371-1. Sci Rep. 2025. PMID: 40360535 Free PMC article.
References
-
- Schuster , Sharynn , et al. Treating enuresis in children with neurodevelopmental disorders using bell and pad alarm. Journal of Pediatric Urology. 2021 - PubMed
-
- Caldwell Patrina Hy, Deshpande Aniruddh V, Von Gontard Alexander. Management of nocturnal enuresis. Bmj. 2013;347 - PubMed
-
- Van De Walle Johan, Van Herzeele Charlotte, Raes Ann. Is There Still a Role for Desmopressin in Children with Primary Monosymptomatic Nocturnal Enuresis? Drug safety. 2010;33.4:261–271. - PubMed
-
- Fockema , Margaret W, et al. Enuresis in South African children: prevalence, associated factors and parental perception of treatment. BJU international. 2012;110.11c:E1114–E1120. - PubMed
-
- Vogt , Mandy , et al. Evaluation of different modes of combined therapy in children with monosymptomatic nocturnal enuresis. BJU international. 2010;105.10:1456–1459. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials