This is a preprint.
Vaccine Protection Against the SARS-CoV-2 Omicron Variant in Macaques
- PMID: 35169798
- PMCID: PMC8845420
- DOI: 10.1101/2022.02.06.479285
Vaccine Protection Against the SARS-CoV-2 Omicron Variant in Macaques
Update in
-
Vaccine protection against the SARS-CoV-2 Omicron variant in macaques.Cell. 2022 Apr 28;185(9):1549-1555.e11. doi: 10.1016/j.cell.2022.03.024. Epub 2022 Mar 17. Cell. 2022. PMID: 35427477 Free PMC article.
Abstract
Background: The rapid spread of the SARS-CoV-2 Omicron (B.1.1.529) variant, including in highly vaccinated populations, has raised important questions about the efficacy of current vaccines. Immune correlates of vaccine protection against Omicron are not known.
Methods: 30 cynomolgus macaques were immunized with homologous and heterologous prime-boost regimens with the mRNA-based BNT162b2 vaccine and the adenovirus vector-based Ad26.COV2.S vaccine. Following vaccination, animals were challenged with the SARS-CoV-2 Omicron variant by the intranasal and intratracheal routes.
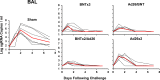
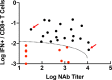
Results: Omicron neutralizing antibodies were observed following the boost immunization and were higher in animals that received BNT162b2, whereas Omicron CD8+ T cell responses were higher in animals that received Ad26.COV2.S. Following Omicron challenge, sham controls showed more prolonged virus in nasal swabs than in bronchoalveolar lavage. Vaccinated macaques demonstrated rapid control of virus in bronchoalveolar lavage, and most vaccinated animals also controlled virus in nasal swabs, showing that current vaccines provide substantial protection against Omicron in this model. However, vaccinated animals that had moderate levels of Omicron neutralizing antibodies but negligible Omicron CD8+ T cell responses failed to control virus in the upper respiratory tract. Virologic control correlated with both antibody and T cell responses.
Conclusions: BNT162b2 and Ad26.COV2.S provided robust protection against high-dose challenge with the SARS-CoV-2 Omicron variant in macaques. Protection against this highly mutated SARS-CoV-2 variant correlated with both humoral and cellular immune responses.
Conflict of interest statement
Competing Interests
DHB is a co-inventor on provisional vaccine patents (63/121,482; 63/133,969; 63/135,182). The authors report no other conflict of interest. ACMB has received funding from Abbvie for the commercial development of SARS-CoV-2 mAbs.
Figures







References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
