This is a preprint.
Antibody and memory B-cell immunity in a heterogeneously SARS-CoV-2 infected and vaccinated population
- PMID: 35169812
- PMCID: PMC8845433
- DOI: 10.1101/2022.02.07.22270626
Antibody and memory B-cell immunity in a heterogeneously SARS-CoV-2 infected and vaccinated population
Update in
-
Antibody and Memory B-Cell Immunity in a Heterogeneously SARS-CoV-2-Infected and -Vaccinated Population.mBio. 2022 Aug 30;13(4):e0084022. doi: 10.1128/mbio.00840-22. Epub 2022 Jun 23. mBio. 2022. PMID: 35735743 Free PMC article.
Abstract
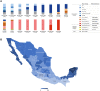
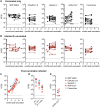
Global population immunity to SARS-CoV-2 is accumulating through heterogenous combinations of infection and vaccination. Vaccine distribution in low- and middle-income countries has been variable and reliant on diverse vaccine platforms. We studied B-cell immunity in Mexico, a middle-income country where five different vaccines have been deployed to populations with high SARS-CoV-2 incidence. Levels of antibodies that bound a stabilized prefusion spike trimer, neutralizing antibody titers and memory B-cell expansion correlated with each other across vaccine platforms. Nevertheless, the vaccines elicited variable levels of B-cell immunity, and the majority of recipients had undetectable neutralizing activity against the recently emergent omicron variant. SARS-CoV-2 infection, experienced prior to or after vaccination potentiated B-cell immune responses and enabled the generation of neutralizing activity against omicron and SARS-CoV for all vaccines in nearly all individuals. These findings suggest that broad population immunity to SARS-CoV-2 will eventually be achieved, but by heterogenous paths.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
