Miniature mass spectrometer-based point-of-care assay for cabotegravir and rilpivirine in whole blood
- PMID: 35169905
- PMCID: PMC9018536
- DOI: 10.1007/s00216-022-03954-3
Miniature mass spectrometer-based point-of-care assay for cabotegravir and rilpivirine in whole blood
Abstract
HIV prevention and treatment with injectable cabotegravir and/or rilpivirine administered once every 4 to 8 weeks is an attractive alternative to daily therapy. Prescribed dosage and drug concentrations in plasma are based on patient data collected in clinical trials, but actual patients are expected to exhibit more variability in drug concentrations, which is important to quantify. Here, we demonstrate the first quantitative point-of-care assay with a miniature mass spectrometer to assess these drug concentrations in whole blood. Quantitative performance is obtained using paper spray ionization in combination with tandem mass spectrometry (MS/MS) in the clinically relevant concentration range of both drugs. Limits of quantitation (LoQs) of cabotegravir and rilpivirine are measured to be 750 ng/mL and 20 ng/mL, respectively. The assay turnaround time is < 4 min, and strong linear relationships are established between MS/MS responses and concentration, with percentage of relative standard deviations (RSDs) that are <15% at concentrations above the LoQs. The speed, portability, low power consumption, and specificity offered by the miniature instrument should make it an appropriate platform for measuring drug concentrations in a walk-in clinic using small volumes of patient blood.
Keywords: Ambient ionization; Antiretrovirals; Drug quantitation; HIV treatment; Paper spray.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
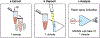

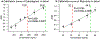
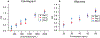
References
-
- UNAIDS Global HIV & AIDS statistics — Fact sheet ∣ UNAIDS. In: UNAIDS 2021 Epidemiol. Estim 2021. https://www.unaids.org/en/resources/fact-sheet. Accessed 23 Sep 2021
-
- U.S. Food & drug administration FDA Approves Cabenuva and Vocabria for the Treatment of HIV-1 Infection. 2021. https://www.fda.gov/drugs/human-immunodeficiency-virus-hiv/fda-approves-.... Accessed 29 Sep 2021
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

