Electrochemical immunosensors using electrodeposited gold nanostructures for detecting the S proteins from SARS-CoV and SARS-CoV-2
- PMID: 35169906
- PMCID: PMC8853172
- DOI: 10.1007/s00216-022-03956-1
Electrochemical immunosensors using electrodeposited gold nanostructures for detecting the S proteins from SARS-CoV and SARS-CoV-2
Abstract
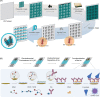
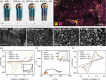
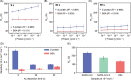
This paper reports the development of a low-cost (< US$ 0.03 per device) immunosensor based on gold-modified screen-printed carbon electrodes (SPCEs). As a proof of concept, the immunosensor was tested for a fast and sensitive determination of S proteins from both SARS-CoV and SARS-CoV-2, by a single disposable device. Gold nanoparticles were electrochemically deposited via direct reduction of gold ions on the electrode using amperometry. Capture antibodies from spike (S) protein were covalently immobilized on carboxylic groups of self-assembled monolayers (SAM) of mercaptoacetic acid (MAA) attached to the gold nanoparticles. Label-free detection of S proteins from both SARS-CoV and SARS-CoV-2 was performed with electrochemical impedance spectroscopy (EIS). The immunosensor fabricated with 9 s gold deposition had a high performance in terms of selectivity, sensitivity, and low limit of detection (LOD) (3.16 pmol L-1), thus permitting the direct determination of the target proteins in spiked saliva samples. The complete analysis can be carried out within 35 min using a simple one-step assay protocol with small sample volumes (10 µL). With such features, the immunoplatform presented here can be deployed for mass testing in point-of-care settings.
Keywords: Diagnosis; Gold nanoparticles; Immunosensor; S protein; SARS-CoV; SARS-CoV-2.
© 2022. Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Brazaca LC, dos Santos PL, de Oliveira PR, Rocha DP, Stefano JS, Kalinke C, Abarza Muñoz RA, Bonacin JA, Janegitz BC, Carrilho E. Biosensing strategies for the electrochemical detection of viruses and viral diseases – a review. Anal Chim Acta. 2021;1159:338384. doi: 10.1016/j.aca.2021.338384. - DOI - PMC - PubMed
-
- World Health Organization (2020) Coronavirus disease 2019 (COVID-19): situation report, 52. https://apps.who.int/iris/handle/10665/331476. Accessed 12 Mar 2021
MeSH terms
Substances
Grants and funding
- 164569/2020-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 303338/2019-9/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 315824/2020-4/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 423952/2018-8/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 465389/2014-7/Conselho Nacional de Desenvolvimento Científico e Tecnológico
- 2014/40867-3/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2016/01919-6/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2017/05362-9/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2017/21097-3/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2018/19750-3/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2018/22214-6/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2019/01777-5/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 2020/09587-8/Fundação de Amparo à Pesquisa do Estado de São Paulo
- 3007/2014 - PROCAD/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- 88887.504861/2020-00/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
- Finance Code 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

