Prevention of arterial and venous thrombotic events in symptomatic peripheral arterial disease patients after lower extremity revascularization in the VOYAGER PAD trial: Dual anticoagulant/antiplatelet regimen vs antiplatelet therapy alone
- PMID: 35170216
- PMCID: PMC9314576
- DOI: 10.1111/jth.15673
Prevention of arterial and venous thrombotic events in symptomatic peripheral arterial disease patients after lower extremity revascularization in the VOYAGER PAD trial: Dual anticoagulant/antiplatelet regimen vs antiplatelet therapy alone
Abstract
Background: Vascular disease burden after lower extremity revascularization (LER) comprises more than the first event, more vascular beds than the local arteries, and more than one clinical event type.
Objectives: Assess total arterial and venous thrombotic burden after LER for symptomatic peripheral artery disease (PAD) and effect of low-dose anticoagulation added to low-dose antiplatelet therapy.
Patients/methods: VOYAGER PAD randomized 6564 symptomatic PAD patients undergoing LER to rivaroxaban 2.5 mg twice-daily or placebo on aspirin background. Marginal proportional-hazards models used to generate treatment hazard ratios and associated 95% CIs for first and total events; non-thrombotic deaths treated as competing terminal events. Incidence rates calculated as number of events per 100 patient-years follow-up.
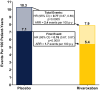
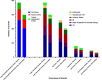
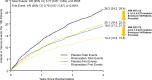
Results: Over 2.5 years (median), first and total thrombotic event rates: 7.1 and 10.3 events/100 patient-years, respectively, in placebo group. Two-thirds (925/1372) of total thrombotic events (arterial 95%, venous 5%) were nonfatal first events. Nearly one-third of patients with first event had a second arterial or venous thrombotic event. Rivaroxaban plus aspirin reduced first and total arterial and venous thrombotic events to 5.4 and 7.9 events/100 patient-years, respectively, a reduction in total thrombotic events over aspirin of 23% (HR: 0.77, 95%CI: 0.67-0.89, p = .0005), preventing 6.1 total arterial and venous thrombotic events at 3 years.
Conclusions: Assessing total arterial and venous thrombotic events, not just first events, provides more complete information about disease burden and absolute on-treatment impact. Following LER, judicious modulation of more than one coagulation pathway can provide broader benefit than intensifying inhibition of one hemostatic system component.
Keywords: anticoagulants; atherosclerosis; peripheral arterial disease; rivaroxaban; thrombosis; venous thromboembolism.
© 2022 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
Drs. Berkowitz, Nehler, Hsia, Capell, Hess, Hsia, and Bonaca report that their University of Colorado salary is partially supported through funds to the University from the Colorado Prevention Center, a non‐profit academic research organization affiliated with the University of Colorado, that receives research grant/consulting funding from: Abbott, Agios, Alexion Pharma, Alnylam, Amgen, Angionetics, ARCA Biopharma, Array, AstraZeneca, Atentiv, Audentes, Bayer, Better Therapeutics, Brigham and Women's Hospital, Bristol‐Myers Squibb, Cardiol Therapeutics, CellResearch, Cook Medical, Cook, CSL Behring, Eidos Therapeutics, EP Trading Co, Esperion Therapeutics, Everly Health, Faraday, Fortress Biotech, HDL Therapeutics, Heartflow, Hummingbird Bioscience, Insmed, Janssen, Kowa Research, Lexicon, Merck, MedPace, Medtronic, Moderna, Novate Medical, NovoNordisk, Pfizer, PhaseBio, PPD Development, Prairie Education and Research, Prothena Biosciences, Regeneron, Regio Biosciences, Sanifit Therapeutics, Sanofi, Smith and Nephew, Stealth BioTherapeutics, University of Colorado, University of Pittsburgh, Worldwide Clinical Trials, Wraser, Yale Cardiovascular Research Group.
Dr. Bauersachs reports consultation and speaker´s honoraria from Aspen, Bayer, Bristol Myers Squibb and Pfizer.
Dr. Szarek reports grant support from Resverlogix, Baxter, and Janssen; Personal fees from CiVi and Esperion; Grant support, personal fees, and non‐financial support from Sanofi; and grant support and non‐financial support from Regeneron.
Dr. Debus reports grants and personal fees from Bayer AG, grants from Cook LTD, grants from Terumo Aortic, during the conduct of the study.
Dr. Patel reports receiving Advisory Board/Consultant Fees: Bayer, Janssen, Heartflow, Novartis, Phillips and Research Grants from Bayer, Janssen, and Heartflow.
Dr. Anand discloses receiving lecture fees from Bayer and Janssen.
Dr. Leeper reports having has received consulting fees from Janssen within the last 2 years.
Dr. Brasil reports the following:
- o
Employee at:
- ▪
Assistant Professor of Medicine, Faculdade de Ciências Médicas de Minas Gerais FCMMG/FELUMA School of Medicine. Principal Investigator in the Centro de Investigacao Clinica (CIC) at Hospital Universitario Ciencias Medicas (HUCM). Belo Horizonte ‐ MG, Brazil.
- ▪
Assistant Professor of Medicine, Faculdade de Ciências da Saúde (FCS), Departamento de Medicina (DME), Universidade Federal de Lavras (UFLA) School of Medicine. Lavras ‐ MG, Brazil.
- ▪
- o
His institution Faculdade de Ciencias Medicas de Minas Gerais FCMMG/FELUMA School of Medicine received payments to conduct Voyager‐PAD from the sponsor (Bayer). DPB served as the NLI for Brazil during Voyager‐PAD.
- o
Institutional grants from BAYER during the conduct of the Voyager PAD study.
- o
Personal fees outside the submitted work from:
- ▪
LIBBS (Brazil) and SERVIER (Brazil) to write scientific educational materials and speak in meetings organized by those pharmaceutical companies.
- ▪
LIBBS (Brazil) to function as member of consulting boards in hypertension, hyperlipidemias, diabetes, and peripheral artery disease.
- ▪
VIATRIS (Brazil) and BIOLAB (Brazil) to function as speaker in scientific meetings.
- ▪
SERVIER (Brazil) to serve as a scientific consultant, deliver interview, and function as member of a consulting board in hypertension.
- ▪
- o
Sponsored in transport and/or hotel accommodations to attend national or international scientific congresses by SERVIER.
- o
Dr. Brasil does not hold any patents, whether planned, pending or issued, broadly relevant to this work.
- o
Dr. Lajos Mátyás notes no conflicts of interest to declare.
Dr. Diaz reports Bayer support via a grant for a trial in Argentina.
Dr. Brodmann reports no conflicts of interest to declare.
Dr. Muehlhofer reports being employed a Bayer AG employee.
Dr. Haskell, being employed by Janssen Pharmaceuticals and owning stock in Johnson & Johnson.
Figures


References
-
- Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral artery disease: results from TRA2{degrees}P‐TIMI 50. Circulation. 2013;127:1522‐1529. - PubMed
-
- Creager MA, Kaufman JA, Conte MS. Acute limb ischemia. N Engl J Med. 2012;366:2198‐2206. - PubMed
-
- Narula N, Dannenberg AJ, Olin JW, et al. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol. 2018;72:2152‐2163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

