New Insight into Dearomatization and Decarbonylation of Antitubercular 4H-Benzo[e][1,3]thiazinones: Stable 5H- and 7H-Benzo[e][1,3]thiazines
- PMID: 35170242
- PMCID: PMC9306624
- DOI: 10.1002/cmdc.202200021
New Insight into Dearomatization and Decarbonylation of Antitubercular 4H-Benzo[e][1,3]thiazinones: Stable 5H- and 7H-Benzo[e][1,3]thiazines
Abstract
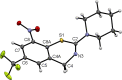
8-Nitro-4H-benzo[e][1,3]thiazinones (BTZs) are potent in vitro antimycobacterial agents. New chemical transformations, viz. dearomatization and decarbonylation, of two BTZs and their influence on the compounds' antimycobacterial properties are described. Reactions of 8-nitro-2-(piperidin-1-yl)-6-(trifluoromethyl)-4H-benzo[e][1,3]thiazin-4-one and the clinical drug candidate BTZ043 with the Grignard reagent CH3 MgBr afford the corresponding dearomatized stable 4,5-dimethyl-5H- and 4,7-dimethyl-7H-benzo[e][1,3]thiazines. These methine compounds are structurally characterized by X-ray crystallography for the first time. Reduction of the BTZ carbonyl group, leading to the corresponding markedly non-planar 4H-benzo[e][1,3]thiazine systems, is achieved using the reducing agent (CH3 )2 S ⋅ BH3 . Double methylation with dearomatization and decarbonylation renders the two BTZs studied inactive against Mycobacterium tuberculosis and Mycobacterium smegmatis, as proven by in vitro growth inhibition assays.
Keywords: BTZ043; DprE1 inhibitors; benzothiazines; benzothiazinones; tuberculosis.
© 2022 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- None
-
- Makarov V., Manina G., Mikusova K., Möllmann U., Ryabova O., Saint-Joanis B., Dhar N., Pasca M. R., Buroni S., Lucarelli A. P., Milano A., De Rossi E., Belanova M., Bobovska A., Dianiskova P., Kordulakova J., Sala C., Fullam E., Schneider P., McKinney J. D., Brodin P., Christophe T., Waddell S., Butcher P., Albrethsen J., Rosenkrands I., Brosch R., Nandi V., Bharath S., Gaonkar S., Shandil R. K., Balasubramanian V., Balganesh T., Tyagi S., Grosset J., Riccardi G., Cole S. T., Science 2009, 324, 801–804; - PMC - PubMed
-
- I. Rudolph, P. Imming, A. Richter, DE102014012546, Martin-Luther-Universitaet Halle-Wittenberg, Germany, 2014.
-
- Makarov V., Mikušová K., Appl. Sci. 2020, 10, 2269.
-
- None
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

