A survey: Understanding the health and perspectives of people with CF not benefiting from CFTR modulators
- PMID: 35170259
- PMCID: PMC9314897
- DOI: 10.1002/ppul.25859
A survey: Understanding the health and perspectives of people with CF not benefiting from CFTR modulators
Abstract
Background: While the advent of cystic fibrosis transmembrane conductance regulator (CFTR) modulator use has improved daily life and long-term prognosis of CF for many with approved CFTR mutations, approximately 10% of people with CF (pwCF) have only symptomatic treatments available.
Methods: Between June 10 and July 1, 2021, Emily's Entourage distributed a 38-question anonymous survey targeted at pwCF not benefitting from approved modulators via social media and email to pwCF and CF advocacy groups in and outside the United States regarding health status, impact of CF, unmet needs, and clinical research interest.
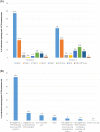
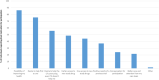
Results: There were 431 survey respondents representing pwCF on five continents. The majority of pwCF had moderate lung disease (50.3%). Ineligibility based on CFTR mutation (64.1%) was the most frequently reported reason pwCF were not on modulators. PwCF reported the most impacted aspects of life were mental (66.7%) and physical (40.7%) health. Financial concerns and feelings of isolation were commonly reported. Witnessing improvements for peers with access to modulators was both uplifting and disheartening. The majority of pwCF would be interested in participating in future clinical research (77.6%), although some living outside of the United States cited lack of opportunity to participate in clinical trials as a barrier.
Conclusions: PwCF who are ineligible, intolerant, or lack access to modulators have a high burden of disease impacting their physical and mental health. Although most are happy for those who are benefiting from modulators, they are eager for the opportunity to experience similar improvements for themselves, and willing to participate in clinical trials of new therapies.
Keywords: 10%; CFTR modulator; cystic fibrosis; nonsense mutation; orphan disease.
© 2022 The Authors. Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
Emily Kramer‐Golinkoff is a founding member of Emily's Entourage. In the past 3 years, her financial and commercial interests were with Medidata Solutions, Inc and Translate Bio, in terms of speaking at meetings/travel. Her noncommercial interests included consulting for the University of Pennsylvania Health System and speaking at meetings/travel from Cystic Fibrosis Research Incorporated, Personalized Medicine Coalition, Academy Health, and BIO International. Amanda Camacho was employed by Emily's Entourage at the time of the design and conduct of the survey as well as at the time of the writing of the original version of the manuscript. Liza Kramer had commercial interests (past 3 years) in terms of speaking at meetings/travel for Translate Bio, Cystic Fibrosis Research Incorporated, and Personalized Medicine Coalition. Jennifer L. Taylor‐Cousar, in the past 3 years, has displayed her financial and commercial interests as faculty for an institution that is part of the CF TDN. She had been site PI on studies for Vertex, Bayer, Celtaxys, Eloxx, Nivalis, and Proteostasis; on advisory boards for Genentech, Gilead, AbbVie, Insmed, and Vertex; done consulting/provided clinical trial design advice for Vertex, Celtaxys, Proteostasis, Santhera, 4DMT, and Polarean; and served on the data monitoring committee for AbbVie. She is a professional member of the CFF Clinical Research Executive Committee, CF TDN Women's Health Research Working Group, and ATS Clinical Problems Programming and Scientific Grant Review Committees. On a noncommercial basis, she had received a grant from the CFF. She also has nonprofit relationships with the CFF Board of Trustees and Emily's Entourage Scientific Advisory Board.
Figures

References
-
- Castellani C, Duff AJA, Bell SC, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17(2):153‐178. - PubMed
-
- Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor‐ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373(18):1783‐1784. - PubMed
-
- Taylor‐Cousar JL, Munck A, McKone EF, et al. Tezacaftor‐ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377(21):2013‐2023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

