Intestinal secretory mechanisms and diarrhea
- PMID: 35170355
- PMCID: PMC8917926
- DOI: 10.1152/ajpgi.00316.2021
Intestinal secretory mechanisms and diarrhea
Abstract
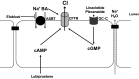
One of the primary functions of the intestinal epithelium is to transport fluid and electrolytes to and from the luminal contents. Under normal circumstances, absorptive and secretory processes are tightly regulated such that absorption predominates, thereby enabling conservation of the large volumes of water that pass through the intestine each day. However, in conditions of secretory diarrhea, this balance becomes dysregulated, so that fluid secretion, driven primarily by Cl- secretion, overwhelms absorptive capacity, leading to increased loss of water in the stool. Secretory diarrheas are common and include those induced by pathogenic bacteria and viruses, allergens, and disruptions to bile acid homeostasis, or as a side effect of many drugs. Here, we review the cellular and molecular mechanisms by which Cl- and fluid secretion in the intestine are regulated, how these mechanisms become dysregulated in conditions of secretory diarrhea, currently available and emerging therapeutic approaches, and how new strategies to exploit intestinal secretory mechanisms are successfully being used in the treatment of constipation.
Keywords: chloride secretion; diarrhea; epithelial transport.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

