Rapid Inhibitor Discovery by Exploiting Synthetic Lethality
- PMID: 35170959
- PMCID: PMC9012225
- DOI: 10.1021/jacs.1c12697
Rapid Inhibitor Discovery by Exploiting Synthetic Lethality
Abstract
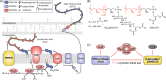
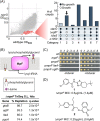
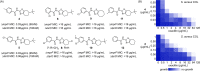
Synthetic lethality occurs when inactivation of two genes is lethal but inactivation of either single gene is not. This phenomenon provides an opportunity for efficient compound discovery. Using differential growth screens, one can identify biologically active compounds that selectively inhibit proteins within the synthetic lethal network of any inactivated gene. Here, based purely on synthetic lethalities, we identified two compounds as the only possible inhibitors of Staphylococcus aureus lipoteichoic acid (LTA) biosynthesis from a screen of ∼230,000 compounds. Both compounds proved to inhibit the glycosyltransferase UgtP, which assembles the LTA glycolipid anchor. UgtP is required for β-lactam resistance in methicillin-resistant S. aureus (MRSA), and the inhibitors restored sensitivity to oxacillin in a highly resistant S. aureus strain. As no other compounds were pursued as possible LTA glycolipid assembly inhibitors, this work demonstrates the extraordinary efficiency of screens that exploit synthetic lethality to discover compounds that target specified pathways. The general approach should be applicable not only to other bacteria but also to eukaryotic cells.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Brown S.; Xia G.; Luhachack L. G.; Campbell J.; Meredith T. C.; Chen C.; Winstel V.; Gekeler C.; Irazoqui J. E.; Peschel A.; Walker S. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 18909–18914. 10.1073/pnas.1209126109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

