Comparison between Nasal and Nasopharyngeal Swabs for SARS-CoV-2 Rapid Antigen Detection in an Asymptomatic Population, and Direct Confirmation by RT-PCR from the Residual Buffer
- PMID: 35171010
- PMCID: PMC8849095
- DOI: 10.1128/spectrum.02455-21
Comparison between Nasal and Nasopharyngeal Swabs for SARS-CoV-2 Rapid Antigen Detection in an Asymptomatic Population, and Direct Confirmation by RT-PCR from the Residual Buffer
Abstract
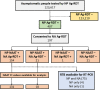
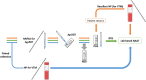
Containment measures employed during the COVID-19 pandemic included prompt recognition of cases, isolation, and contact tracing. Bilateral nasal (NA) swabs applied to a commercial antigen-based rapid diagnostic test (Ag-RDT) offer a simpler and more comfortable alternative to nasopharyngeal (NP) collection; however, little is known about the sensitivity of this method in an asymptomatic population. Participants in community-based asymptomatic testing sites were screened for SARS-CoV-2 using an Ag-RDT with NP sampling. Positive individuals returned for confirmatory molecular testing and consented to repeating the Ag-RDT using a bilateral NA swab for comparison. Residual test buffer (RTB) from Ag-RDTs was subjected to real-time reverse transcription-PCR (RT-PCR). Of 123,617 asymptomatic individuals, 197 NP Ag-RDT-positive participants were included, with 175 confirmed positive by RT-PCR. Of these cases, 154 were identified from the NA swab collection with Ag-RDT, with a sensitivity of 88.0% compared to the NP swab collection. Stratifying results by RT-PCR cycle threshold demonstrated that sensitivity of the nasal collection method varied based on the cycle threshold (CT) value of the paired RT-PCR sample. RT-PCR testing on the RTB from the Ag-RDT using NP and NA swab collections resulted in 100.0% and 98.7% sensitivity, respectively. NA swabs provide an adequate alternative to NP swab collection for use with Ag-RDT, with the recognition that the test is most sensitive in specimens with high viral loads. With the high sensitivity of RT-PCR testing on RTB from Ag-RDT, a more streamlined approach to confirmatory testing is possible without recollection or use of paired collections strategies. IMPORTANCE Nasal swabbing for SARS-CoV-2 (COVID-19) comes with many benefits but is slightly less sensitive than traditional nasopharyngeal swabbing; however, confirmatory lab-based testing could be performed directly from the residual buffer from either sample type.
Keywords: COVID-19; SARS-CoV-2; antigen; nasal; nasopharyngeal; rapid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sensitivity and Specificity of SARS-CoV-2 Rapid Antigen Detection Tests Using Oral, Anterior Nasal, and Nasopharyngeal Swabs: a Diagnostic Accuracy Study.Microbiol Spectr. 2022 Feb 23;10(1):e0202921. doi: 10.1128/spectrum.02029-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107327 Free PMC article.
-
Bean Extract-Based Gargle for Efficient Diagnosis of Active COVID-19 Infection Using Rapid Antigen Tests.Microbiol Spectr. 2022 Feb 23;10(1):e0161421. doi: 10.1128/spectrum.01614-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171037 Free PMC article.
-
Head-to-head performance comparison of self-collected nasal versus professional-collected nasopharyngeal swab for a WHO-listed SARS-CoV-2 antigen-detecting rapid diagnostic test.Med Microbiol Immunol. 2021 Aug;210(4):181-186. doi: 10.1007/s00430-021-00710-9. Epub 2021 May 24. Med Microbiol Immunol. 2021. PMID: 34028625 Free PMC article.
-
Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis.PLoS Med. 2021 Aug 12;18(8):e1003735. doi: 10.1371/journal.pmed.1003735. eCollection 2021 Aug. PLoS Med. 2021. PMID: 34383750 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
Cited by
-
Taqman PACMAN: a simple molecular approach for positive rapid antigen test confirmation during periods of low prevalence.Microbiol Spectr. 2024 May 2;12(5):e0407323. doi: 10.1128/spectrum.04073-23. Epub 2024 Apr 3. Microbiol Spectr. 2024. PMID: 38567975 Free PMC article.
-
Current Advances in Paper-Based Biosensor Technologies for Rapid COVID-19 Diagnosis.Biochip J. 2022;16(4):376-396. doi: 10.1007/s13206-022-00078-9. Epub 2022 Aug 10. Biochip J. 2022. PMID: 35968255 Free PMC article. Review.
-
COVID-19 isolation strategies: What have we learned.Travel Med Infect Dis. 2022 Sep-Oct;49:102416. doi: 10.1016/j.tmaid.2022.102416. Epub 2022 Aug 8. Travel Med Infect Dis. 2022. PMID: 35952965 Free PMC article.
-
The Diagnostic Accuracy of SARS-CoV-2 Nasal Rapid Antigen Self-Test: A Systematic Review and Meta-Analysis.Life (Basel). 2023 Jan 19;13(2):281. doi: 10.3390/life13020281. Life (Basel). 2023. PMID: 36836639 Free PMC article. Review.
-
Designing Sandwich ELISA with Broadly Reactive Anti-Nucleocapsid Monoclonal Antibodies to Detect Bat-Borne Merbecoviruses.Viruses. 2025 Jun 24;17(7):886. doi: 10.3390/v17070886. Viruses. 2025. PMID: 40733504 Free PMC article.
References
-
- LeBlanc JJ, Gubbay JB, Li Y, Needle R, Arneson SR, Marcino D, Charest H, Desnoyers G, Dust K, Fattouh R, Garceau R, German G, Hatchette TF, Kozak RA, Krajden M, Kuschak T, Lang ALS, Levett P, Mazzulli T, McDonald R, Mubareka S, Prystajecky N, Rutherford C, Smieja M, Yu Y, Zahariadis G, Zelyas N, Bastien N, COVID-19 Pandemic Diagnostics Investigation Team of the Canadian Public Health Laboratory Network (CPHLN) Respiratory Virus Working Group. 2020. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol 128:104433. doi:10.1016/j.jcv.2020.104433. - DOI - PMC - PubMed
-
- Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. 2021. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev 34:e00228-20. doi:10.1128/CMR.00228-20. - DOI - PMC - PubMed
-
- Patriquin G, Davidson RJ, Hatchette TF, Head BM, Mejia E, Becker MG, Meyers A, Sandstrom P, Hatchette J, Block A, Smith N, Ross J, LeBlanc JJ. 2021. Generation of false-positive SARS-CoV-2 antigen results with testing conditions outside manufacturer recommendations: a scientific approach to pandemic misinformation. Microbiol Spectr 9:15. doi:10.1128/Spectrum.00683-21. - DOI - PMC - PubMed
-
- Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes MÁ, Martínez M, Poujois S, Forqué L, Valdivia A, Solano de la Asunción C, Ferrer J, Colomina J, Navarro D. 2020. Field evaluation of a rapid antigen test (Panbio COVID-19 Ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect 27:472.e7–472.e10. - PMC - PubMed
-
- Linares M, Pérez-Tanoira R, Carrero A, Romanyk J, Pérez-García F, Gómez-Herruz P, Arroyo T, Cuadros J. 2020. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J Clin Virol 133:104659. doi:10.1016/j.jcv.2020.104659. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

