Isolation and Characterization of Novel Lytic Phages Infecting Multidrug-Resistant Escherichia coli
- PMID: 35171030
- PMCID: PMC8849078
- DOI: 10.1128/spectrum.01678-21
Isolation and Characterization of Novel Lytic Phages Infecting Multidrug-Resistant Escherichia coli
Abstract
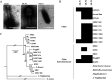
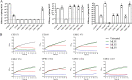
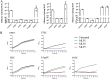
Urinary tract infections (UTIs) are the second most frequent bacterial infections worldwide, with Escherichia coli being the main causative agent. The increase of antibiotic-resistance determinants among isolates from clinical samples, including UTIs, makes the development of novel therapeutic strategies a necessity. In this context, the use of bacteriophages as a therapeutic alternative has been proposed, due to their ability to efficiently kill bacteria. In this work, we isolated and characterized three novel bacteriophages, microbes laboratory phage 1 (MLP1), MLP2, and MLP3, belonging to the Chaseviridae, Myoviridae, and Podoviridae families, respectively. These phages efficiently infect and kill laboratory reference strains and multidrug-resistant clinical E. coli isolates from patients with diagnosed UTIs. Interestingly, these phages are also able to infect intestinal pathogenic Escherichia coli strains, such as enteroaggregative E. coli and diffusely adherent E. coli. Our data show that the MLP phages recognize different regions of the lipopolysaccharide (LPS) molecule, an important virulence factor in bacteria that is also highly variable among different E. coli strains. Altogether, our results suggest that these phages may represent an interesting alternative for the treatment of antibiotic-resistant E. coli. IMPORTANCE Urinary tract infections affect approximately 150 million people annually. The current antibiotic resistance crisis demands the development of novel therapeutic alternatives. Our results show that three novel phages, MLP1, MLP2, and MLP3 are able to infect both laboratory and multidrug-resistant clinical isolates of Escherichia coli. Since these phages (i) efficiently kill antibiotic-resistant clinical isolates of uropathogenic Escherichia coli (UPEC), (ii) recognize different portions of the LPS molecule, and (iii) are able to efficiently infect intestinal pathogenic Escherichia coli hosts, we believe that these novel phages are good candidates to be used as a therapeutic alternative to treat antibiotic-resistant E. coli strains generating urinary tract and/or intestinal infections.
Keywords: Escherichia coli; LPS; antibiotic resistance; antibiotic-resistant pathogens; bacteriophages; lipopolysaccharide; multidrug-resistant clinical isolates; phage predation; phage-host interactions; urinary tract infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Genomic and proteomic characterization of four novel Schitoviridae family phages targeting uropathogenic Escherichia coli strain.Virol J. 2025 Mar 21;22(1):83. doi: 10.1186/s12985-025-02691-0. Virol J. 2025. PMID: 40119445 Free PMC article.
-
|Isolation and characterization of novel bacteriophages as a potential therapeutic option for Escherichia coli urinary tract infections.Appl Microbiol Biotechnol. 2021 Jul;105(13):5617-5629. doi: 10.1007/s00253-021-11432-6. Epub 2021 Jul 12. Appl Microbiol Biotechnol. 2021. PMID: 34254156 Free PMC article.
-
Characterization of bacteriophages infecting multidrug-resistant uropathogenic Escherichia coli strains.Arch Virol. 2024 Jun 8;169(7):142. doi: 10.1007/s00705-024-06063-x. Arch Virol. 2024. PMID: 38851653 Free PMC article.
-
Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains.Acta Biochim Pol. 2019 May 28;66(2):129-138. doi: 10.18388/abp.2018_2787. Acta Biochim Pol. 2019. PMID: 31136644 Review.
-
Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli.Dan Med Bull. 2011 Apr;58(4):B4187. Dan Med Bull. 2011. PMID: 21466767 Review.
Cited by
-
Isolation and Genomic Analysis of Escherichia coli Phage AUBRB02: Implications for Phage Therapy in Lebanon.Antibiotics (Basel). 2025 Apr 30;14(5):458. doi: 10.3390/antibiotics14050458. Antibiotics (Basel). 2025. PMID: 40426525 Free PMC article.
-
Lytic Spectra of Tailed Bacteriophages: A Systematic Review and Meta-Analysis.Viruses. 2024 Dec 4;16(12):1879. doi: 10.3390/v16121879. Viruses. 2024. PMID: 39772189 Free PMC article.
-
Multi-omics analysis of gut microbiota and metabolites reveals contrasting profiles in domestic pigs and wild boars across urban environments.Front Microbiol. 2024 Aug 13;15:1450306. doi: 10.3389/fmicb.2024.1450306. eCollection 2024. Front Microbiol. 2024. PMID: 39193431 Free PMC article.
-
Rapid formulation of a genetically diverse phage cocktail targeting uropathogenic Escherichia coli infections using the UTI89 model.Sci Rep. 2025 Apr 14;15(1):12832. doi: 10.1038/s41598-025-96561-y. Sci Rep. 2025. PMID: 40229393 Free PMC article.
-
Isolation and Characterization of Lytic Bacteriophages Active against Clinical Strains of E. coli and Development of a Phage Antimicrobial Cocktail.Viruses. 2022 Oct 28;14(11):2381. doi: 10.3390/v14112381. Viruses. 2022. PMID: 36366479 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

