Antimicrobial production by perifollicular dermal preadipocytes is essential to the pathophysiology of acne
- PMID: 35171653
- PMCID: PMC9885891
- DOI: 10.1126/scitranslmed.abh1478
Antimicrobial production by perifollicular dermal preadipocytes is essential to the pathophysiology of acne
Abstract
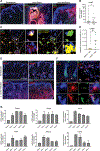
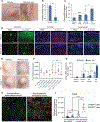
Innate immune defense against deep tissue infection by Staphylococcus aureus is orchestrated by fibroblasts that become antimicrobial when triggered to differentiate into adipocytes. However, the role of this process in noninfectious human diseases is unknown. To investigate the potential role of adipogenesis by dermal fibroblasts in acne, a disorder triggered by Cutibacterium acnes, single-cell RNA sequencing was performed on human acne lesions and mouse skin challenged by C. acnes. A transcriptome consistent with adipogenesis was observed within specific fibroblast subsets from human acne and mouse skin lesions infected with C. acnes. Perifollicular dermal preadipocytes in human acne and mouse skin lesions showed colocalization of PREF1, an early marker of adipogenesis, and cathelicidin (Camp), an antimicrobial peptide. This capacity of C. acnes to specifically trigger production of cathelicidin in preadipocytes was dependent on TLR2. Treatment of wild-type mice with retinoic acid (RA) suppressed the capacity of C. acnes to form acne-like lesions, inhibited adipogenesis, and enhanced cathelicidin expression in preadipocytes, but lesions were unresponsive in Camp-/- mice, despite the anti-adipogenic action of RA. Analysis of inflamed skin of acne patients after retinoid treatment also showed enhanced induction of cathelicidin, a previously unknown beneficial effect of retinoids in difficult-to-treat acne. Overall, these data provide evidence that adipogenic fibroblasts are a critical component of the pathogenesis of acne and represent a potential target for therapy.
Conflict of interest statement
Figures



References
-
- Kovács D, Lovászi M, Póliska S, Oláh A, Bíró T, Veres I, Zouboulis CC, Ståhle M, Rühl R, Remenyik É, Törőcsik D, Sebocytes differentially express and secrete adipokines. Exp. Dermatol. 25, 194–199 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

