ATP-responsive biomolecular condensates tune bacterial kinase signaling
- PMID: 35171683
- PMCID: PMC8849385
- DOI: 10.1126/sciadv.abm6570
ATP-responsive biomolecular condensates tune bacterial kinase signaling
Abstract
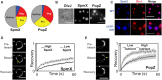
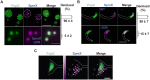
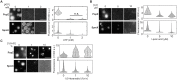
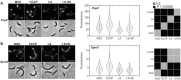
Biomolecular condensates formed via liquid-liquid phase separation enable spatial and temporal organization of enzyme activity. Phase separation in many eukaryotic condensates has been shown to be responsive to intracellular adenosine triphosphate (ATP) levels, although the consequences of these mechanisms for enzymes sequestered within the condensates are unknown. Here, we show that ATP depletion promotes phase separation in bacterial condensates composed of intrinsically disordered proteins. Enhanced phase separation promotes the sequestration and activity of a client kinase enabling robust signaling and maintenance of viability under the stress posed by nutrient scarcity. We propose that a diverse repertoire of condensates can serve as control knobs to tune enzyme sequestration and reactivity in response to the metabolic state of bacterial cells.
Figures



References
-
- Shin Y., Brangwynne C. P., Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017). - PubMed
-
- Patel A., Malinovska L., Saha S., Wang J., Alberti S., Krishnan Y., Hyman A. A., ATP as a biological hydrotrope. Science 356, 753–756 (2017). - PubMed
-
- Mehringer J., Do T. M., Touraud D., Hohenschutz M., Khoshsima A., Horinek D., Kunz W., Hofmeister versus Neuberg: Is ATP really a biological hydrotrope? Cell Rep. Phys. Sci. 2, 100343 (2021).
-
- Govers S. K., Jacobs-Wagner C., Caulobacter crescentus: Model system extraordinaire. Curr. Biol. 30, R1151–R1158 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

