Prescribed medicine use and extent of off-label use according to age in a nationwide sample of Australian children
- PMID: 35172017
- PMCID: PMC9540111
- DOI: 10.1111/ppe.12870
Prescribed medicine use and extent of off-label use according to age in a nationwide sample of Australian children
Abstract
Background: Medicine prescribing for children is impacted by a lack of paediatric-specific dosing, efficacy and safety data for many medicines.
Objectives: To estimate the prevalence of medicine use among children and the rate of 'off-label' prescribing according to age at dispensing.
Methods: We used population-wide primarily outpatient dispensing claims data for 15% of Australian children (0-17 years), 2013-2017 (n = 840,190). We estimated prescribed medicine use and 'off-label' medicine use according to the child's age (<1 year, 1-5 years, 6-11 years, 12-17 years) defined as medicines without age-appropriate dose recommendations in regulator-approved product information. Within off-label medicines, we also identified medicines with and without age-specific dose recommendations in a national prescribing guide, the Australian Medicines Handbook Children's Dosing Companion (AMH CDC).
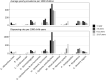
Results: The overall dispensing rate was 2.0 dispensings per child per year. The medicines with the highest average yearly prevalence were systemic antibiotics (435.3 per 1000 children), greatest in children 1-5 years (546.9 per 1000). Other common medicine classes were systemic corticosteroids (92.7 per 1000), respiratory medicines (91.2 per 1000), acid-suppressing medicines in children <1 year (47.2 per 1000), antidepressants in children 12-17 years (40.3 per 1000) and psychostimulants in children 6-11 years (27.0 per 1000). We identified 12.2% of dispensings as off-label based on age, but 66.3% of these had age-specific dosing recommendations in the AMH CDC. Among children <1 year, off-label dispensings were commonly acid-suppressing medicines (35.5%) and topical hydrocortisone (33.1%); in children 6-11 years, off-label prescribing of clonidine (16.0%) and risperidone (13.1%) was common. Off-label dispensings were more likely to be prescribed by a specialist (21.7%) than on-label dispensings (7.5%).
Conclusions: Prescribed medicine use is common in children, with off-label dispensings for medicines without paediatric-specific dosing guidelines concentrated in classes such as acid-suppressing medicines and psychotropics. Our findings highlight a need for better evidence to support best-practice prescribing.
Keywords: Australia; attention deficit disorder with hyperactivity; gastroesophageal reflux; paediatrics; pharmacoepidemiology.
© 2022 The Authors. Paediatric and Perinatal Epidemiology published by John Wiley & Sons Ltd.
Conflict of interest statement
AS, CB, ML, HZ and SAP are employees of the Centre for Big Data Research in Health, UNSW Sydney which received funding in 2020 from AbbVie Australia to conduct research, unrelated to the submitted work. AbbVie did not have any knowledge of, or involvement in, the present study. SAP is a member of the Drug Utilisation Sub Committee of the Pharmaceutical Benefits Advisory Committee. The views expressed in this paper do not represent those of the Committee.
Figures
Comment in
-
Pharmacoepidemiology: A key complementary tool to evaluate the paediatric exposome.Paediatr Perinat Epidemiol. 2022 Sep;36(5):738-740. doi: 10.1111/ppe.12907. Epub 2022 Jul 12. Paediatr Perinat Epidemiol. 2022. PMID: 35821649 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical