Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection
- PMID: 35172051
- PMCID: PMC8908850
- DOI: 10.1056/NEJMoa2118691
Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection
Abstract
Background: The duration and effectiveness of immunity from infection with and vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are relevant to pandemic policy interventions, including the timing of vaccine boosters.
Methods: We investigated the duration and effectiveness of immunity in a prospective cohort of asymptomatic health care workers in the United Kingdom who underwent routine polymerase-chain-reaction (PCR) testing. Vaccine effectiveness (≤10 months after the first dose of vaccine) and infection-acquired immunity were assessed by comparing the time to PCR-confirmed infection in vaccinated persons with that in unvaccinated persons, stratified according to previous infection status. We used a Cox regression model with adjustment for previous SARS-CoV-2 infection status, vaccine type and dosing interval, demographic characteristics, and workplace exposure to SARS-CoV-2.
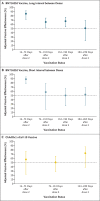
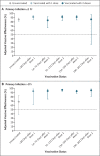
Results: Of 35,768 participants, 27% (9488) had a previous SARS-CoV-2 infection. Vaccine coverage was high: 95% of the participants had received two doses (78% had received BNT162b2 vaccine [Pfizer-BioNTech] with a long interval between doses, 9% BNT162b2 vaccine with a short interval between doses, and 8% ChAdOx1 nCoV-19 vaccine [AstraZeneca]). Between December 7, 2020, and September 21, 2021, a total of 2747 primary infections and 210 reinfections were observed. Among previously uninfected participants who received long-interval BNT162b2 vaccine, adjusted vaccine effectiveness decreased from 85% (95% confidence interval [CI], 72 to 92) 14 to 73 days after the second dose to 51% (95% CI, 22 to 69) at a median of 201 days (interquartile range, 197 to 205) after the second dose; this effectiveness did not differ significantly between the long-interval and short-interval BNT162b2 vaccine recipients. At 14 to 73 days after the second dose, adjusted vaccine effectiveness among ChAdOx1 nCoV-19 vaccine recipients was 58% (95% CI, 23 to 77) - considerably lower than that among BNT162b2 vaccine recipients. Infection-acquired immunity waned after 1 year in unvaccinated participants but remained consistently higher than 90% in those who were subsequently vaccinated, even in persons infected more than 18 months previously.
Conclusions: Two doses of BNT162b2 vaccine were associated with high short-term protection against SARS-CoV-2 infection; this protection waned considerably after 6 months. Infection-acquired immunity boosted with vaccination remained high more than 1 year after infection. (Funded by the U.K. Health Security Agency and others; ISRCTN Registry number, ISRCTN11041050.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Compulsary SARS-CoV-2 (booster-) vaccination in healthcare facilities can not replace personal protection measures while dealing with vulnerable individuals.J Infect. 2022 Jun;84(6):e111. doi: 10.1016/j.jinf.2022.03.019. Epub 2022 Mar 24. J Infect. 2022. PMID: 35339503 Free PMC article. No abstract available.
-
Added Benefit of Covid-19 Vaccination after Previous Infection.N Engl J Med. 2022 Mar 31;386(13):1278-1279. doi: 10.1056/NEJMe2201380. N Engl J Med. 2022. PMID: 35353966 Free PMC article. No abstract available.
-
Protection against SARS-CoV-2 after Vaccination and Previous Infection.N Engl J Med. 2022 Jun 30;386(26):2534. doi: 10.1056/NEJMc2205618. Epub 2022 Jun 15. N Engl J Med. 2022. PMID: 35704417 No abstract available.
References
-
- UK Health Security Agency. COVID-19 — SARS-CoV-2. In: The green book. 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploa...).
-
- Department of Health and Social Care. Optimising the COVID-19 vaccination programme for maximum short-term impact. 2021. (https://www.gov.uk/government/publications/prioritising-the-first-covid-...).
-
- UK Health Security Agency. Coronavirus (COVID-19) in the UK: vaccinations in United Kingdom. 2021. (https://coronavirus.data.gov.uk/details/vaccinations).
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
