Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19
- PMID: 35172054
- PMCID: PMC8908851
- DOI: 10.1056/NEJMoa2118542
Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19
Abstract
Background: Nirmatrelvir is an orally administered severe acute respiratory syndrome coronavirus 2 main protease (Mpro) inhibitor with potent pan-human-coronavirus activity in vitro.
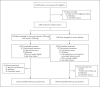
Methods: We conducted a phase 2-3 double-blind, randomized, controlled trial in which symptomatic, unvaccinated, nonhospitalized adults at high risk for progression to severe coronavirus disease 2019 (Covid-19) were assigned in a 1:1 ratio to receive either 300 mg of nirmatrelvir plus 100 mg of ritonavir (a pharmacokinetic enhancer) or placebo every 12 hours for 5 days. Covid-19-related hospitalization or death from any cause through day 28, viral load, and safety were evaluated.
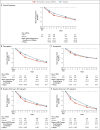
Results: A total of 2246 patients underwent randomization; 1120 patients received nirmatrelvir plus ritonavir (nirmatrelvir group) and 1126 received placebo (placebo group). In the planned interim analysis of patients treated within 3 days after symptom onset (modified intention-to treat population, comprising 774 of the 1361 patients in the full analysis population), the incidence of Covid-19-related hospitalization or death by day 28 was lower in the nirmatrelvir group than in the placebo group by 6.32 percentage points (95% confidence interval [CI], -9.04 to -3.59; P<0.001; relative risk reduction, 89.1%); the incidence was 0.77% (3 of 389 patients) in the nirmatrelvir group, with 0 deaths, as compared with 7.01% (27 of 385 patients) in the placebo group, with 7 deaths. Efficacy was maintained in the final analysis involving the 1379 patients in the modified intention-to-treat population, with a difference of -5.81 percentage points (95% CI, -7.78 to -3.84; P<0.001; relative risk reduction, 88.9%). All 13 deaths occurred in the placebo group. The viral load was lower with nirmatrelvir plus ritonavir than with placebo at day 5 of treatment, with an adjusted mean difference of -0.868 log10 copies per milliliter when treatment was initiated within 3 days after the onset of symptoms. The incidence of adverse events that emerged during the treatment period was similar in the two groups (any adverse event, 22.6% with nirmatrelvir plus ritonavir vs. 23.9% with placebo; serious adverse events, 1.6% vs. 6.6%; and adverse events leading to discontinuation of the drugs or placebo, 2.1% vs. 4.2%). Dysgeusia (5.6% vs. 0.3%) and diarrhea (3.1% vs. 1.6%) occurred more frequently with nirmatrelvir plus ritonavir than with placebo.
Conclusions: Treatment of symptomatic Covid-19 with nirmatrelvir plus ritonavir resulted in a risk of progression to severe Covid-19 that was 89% lower than the risk with placebo, without evident safety concerns. (Supported by Pfizer; ClinicalTrials.gov number, NCT04960202.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
African clinical trial denied access to key COVID drug Paxlovid.Nature. 2022 Apr;604(7906):412-413. doi: 10.1038/d41586-022-00919-5. Nature. 2022. PMID: 35379975 No abstract available.
-
In patients with COVID-19 at risk for severe disease, nirmatrelvir + ritonavir reduced hospitalization or death.Ann Intern Med. 2022 Jun;175(6):JC63. doi: 10.7326/J22-0038. Epub 2022 Jun 7. Ann Intern Med. 2022. PMID: 35667068
-
Nirmatrelvir for Nonhospitalized Adults with Covid-19.N Engl J Med. 2022 Aug 4;387(5):474. doi: 10.1056/NEJMc2206277. Epub 2022 Jul 20. N Engl J Med. 2022. PMID: 35857647 No abstract available.
-
Nirmatrelvir for Nonhospitalized Adults with Covid-19.N Engl J Med. 2022 Aug 4;387(5):474-475. doi: 10.1056/NEJMc2206277. Epub 2022 Jul 20. N Engl J Med. 2022. PMID: 35857648 No abstract available.
-
COVID drug Paxlovid was hailed as a game-changer. What happened?Nature. 2023 Jan;613(7943):224-225. doi: 10.1038/d41586-022-04576-6. Nature. 2023. PMID: 36599997 No abstract available.
-
Generalized use of Nirmatrelvir plus ritonavir (Paxlovid): Raising concerns.Enferm Infecc Microbiol Clin (Engl Ed). 2023 Feb;41(2):127-128. doi: 10.1016/j.eimce.2022.04.014. Enferm Infecc Microbiol Clin (Engl Ed). 2023. PMID: 36759050 Free PMC article. No abstract available.
References
-
- Center for Systems Science and Engineering. COVID-19 Dashboard. Johns Hopkins University (https://coronavirus.jhu.edu/map.html).
-
- Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021;72(9):e206-e214. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
