Effectiveness of the BNT162b2 Vaccine after Recovery from Covid-19
- PMID: 35172072
- PMCID: PMC8908846
- DOI: 10.1056/NEJMoa2119497
Effectiveness of the BNT162b2 Vaccine after Recovery from Covid-19
Abstract
Background: The risk of infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) decreases substantially among patients who have recovered from coronavirus disease 2019 (Covid-19). However, it is unknown how long protective immunity lasts. Current guidelines recommend vaccination of recovered patients even though data regarding vaccine effectiveness in such cases are still limited.
Methods: In this retrospective cohort study, we reviewed electronic medical records from a large health care organization in Israel to assess reinfection rates in patients who had recovered from SARS-CoV-2 infection before any vaccination against Covid-19. We compared reinfection rates among patients who had subsequently received the BNT162b2 vaccine (Pfizer-BioNTech) and those who had not been vaccinated between March 1 and November 26, 2021. We used a Cox proportional-hazards regression model with time-dependent covariates to estimate the association between vaccination and reinfection after adjustment for demographic factors and coexisting illnesses. Vaccine effectiveness was estimated as 1 minus the hazard ratio. In a secondary analysis, we evaluated the vaccine effectiveness of one dose as compared with two doses.
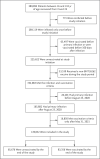
Results: A total of 149,032 patients who had recovered from SARS-CoV-2 infection met the eligibility criteria. Of these patients, 83,356 (56%) received subsequent vaccination during the 270-day study period. Reinfection occurred in 354 of the vaccinated patients (2.46 cases per 100,000 persons per day) and in 2168 of 65,676 unvaccinated patients (10.21 cases per 100,000 persons per day). Vaccine effectiveness was estimated at 82% (95% confidence interval [CI], 80 to 84) among patients who were 16 to 64 years of age and 60% (95% CI, 36 to 76) among those 65 years of age or older. No significant difference in vaccine effectiveness was found for one dose as compared with two doses.
Conclusions: Among patients who had recovered from Covid-19, the receipt of at least one dose of the BNT162b2 vaccine was associated with a significantly lower risk of recurrent infection.
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Added Benefit of Covid-19 Vaccination after Previous Infection.N Engl J Med. 2022 Mar 31;386(13):1278-1279. doi: 10.1056/NEJMe2201380. N Engl J Med. 2022. PMID: 35353966 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination (https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
