Actively implementing an evidence-based feeding guideline for critically ill patients (NEED): a multicenter, cluster-randomized, controlled trial
- PMID: 35172856
- PMCID: PMC8848648
- DOI: 10.1186/s13054-022-03921-5
Actively implementing an evidence-based feeding guideline for critically ill patients (NEED): a multicenter, cluster-randomized, controlled trial
Erratum in
-
Correction to: Actively implementing an evidence-based feeding guideline for critically ill patients (NEED): a multicenter, cluster-randomized, controlled trial.Crit Care. 2022 Apr 21;26(1):115. doi: 10.1186/s13054-022-03982-6. Crit Care. 2022. PMID: 35449019 Free PMC article. No abstract available.
Abstract
Background: Previous cluster-randomized controlled trials evaluating the impact of implementing evidence-based guidelines for nutrition therapy in critical illness do not consistently demonstrate patient benefits. A large-scale, sufficiently powered study is therefore warranted to ascertain the effects of guideline implementation on patient-centered outcomes.
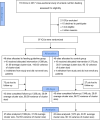
Methods: We conducted a multicenter, cluster-randomized, parallel-controlled trial in intensive care units (ICUs) across China. We developed an evidence-based feeding guideline. ICUs randomly allocated to the guideline group formed a local "intervention team", which actively implemented the guideline using standardized educational materials, a graphical feeding protocol, and live online education outreach meetings conducted by members of the study management committee. ICUs assigned to the control group remained unaware of the guideline content. All ICUs enrolled patients who were expected to stay in the ICU longer than seven days. The primary outcome was all-cause mortality within 28 days of enrollment.
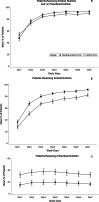
Results: Forty-eight ICUs were randomized to the guideline group and 49 to the control group. From March 2018 to July 2019, the guideline ICUs enrolled 1399 patients, and the control ICUs enrolled 1373 patients. Implementation of the guideline resulted in significantly earlier EN initiation (1.20 vs. 1.55 mean days to initiation of EN; difference - 0.40 [95% CI - 0.71 to - 0.09]; P = 0.01) and delayed PN initiation (1.29 vs. 0.80 mean days to start of PN; difference 1.06 [95% CI 0.44 to 1.67]; P = 0.001). There was no significant difference in 28-day mortality (14.2% vs. 15.2%; difference - 1.6% [95% CI - 4.3% to 1.2%]; P = 0.42) between groups.
Conclusions: In this large-scale, multicenter trial, active implementation of an evidence-based feeding guideline reduced the time to commencement of EN and overall PN use but did not translate to a reduction in mortality from critical illness.
Trial registration: ISRCTN, ISRCTN12233792 . Registered November 20th, 2017.
Keywords: Cluster-randomized trial; Evidence-based guideline; Intensive care unit; Nutrition therapy.
© 2022. The Author(s).
Conflict of interest statement
Dr. Gordon S. Doig reported receiving academic research grants related to nutrition in critical illness from the Australian National Health and Medical Research Council, Fresenius Kabi Deutschland GmbH and Baxter Healthcare Pty Ltd and speakers honoraria from Fresenius Kabi Deutschland GmbH, Baxter Healthcare Australia, Pty Ltd, Nestle Healthcare, Vevy, Switzerland and Nutricia Pharmaceutical (Wuxi) Co., Ltd. China. Dr. van Zanten reports personal fees from Baxter, personal fees from Nestle, personal fees from Fresenius Kabi, grants and personal fees from Nutricia, grants from Cardinal Health, grants from Mermaid, grants from Lyric, outside the submitted work. Dr. Weiqin Li reported receiving speakers honoraria from Nutricia Pharmaceutical (Wuxi) Co., Ltd. China. The remaining authors have disclosed that they do not have any conflicts of interest.
Figures

References
-
- McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. - DOI - PubMed
-
- Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380–398. doi: 10.1007/s00134-016-4665-0. - DOI - PMC - PubMed
-
- Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, Dobb G. Nutrition Guidelines Investigators of the ACTG: Effect of evidence-based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA. 2008;300(23):2731–2741. doi: 10.1001/jama.2008.826. - DOI - PubMed
-
- Heyland DK, Murch L, Cahill N, McCall M, Muscedere J, Stelfox HT, Bray T, Tanguay T, Jiang X, Day AG. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med. 2013;41(12):2743–2753. doi: 10.1097/CCM.0b013e31829efef5. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

