Is it possible to implement a rare disease case-finding tool in primary care? A UK-based pilot study
- PMID: 35172857
- PMCID: PMC8848904
- DOI: 10.1186/s13023-022-02216-w
Is it possible to implement a rare disease case-finding tool in primary care? A UK-based pilot study
Abstract
Introduction: This study implemented MendelScan, a primary care rare disease case-finding tool, into a UK National Health Service population. Rare disease diagnosis is challenging due to disease complexity and low physician awareness. The 2021 UK Rare Diseases Framework highlights as a key priority the need for faster diagnosis to improve clinical outcomes.
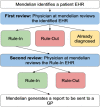
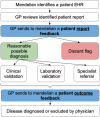
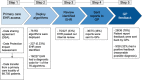
Methods and results: A UK primary care locality with 68,705 patients was examined. MendelScan encodes diagnostic/screening criteria for multiple rare diseases, mapping clinical terms to appropriate SNOMED CT codes (UK primary care standardised clinical terminology) to create digital algorithms. These algorithms were applied to a pseudo-anonymised structured data extract of the electronic health records (EHR) in this locality to "flag" at-risk patients who may require further evaluation. All flagged patients then underwent internal clinical review (a doctor reviewing each EHR flagged by the algorithm, removing all cases with a clear diagnosis/diagnoses that explains the clinical features that led to the patient being flagged); for those that passed this review, a report was returned to their GP. 55 of 76 disease criteria flagged at least one patient. 227 (0.33%) of the total 68,705 of EHR were flagged; 18 EHR were already diagnosed with the disease (the highlighted EHR had a diagnostic code for the same RD it was screened for, e.g. Behcet's disease algorithm identifying an EHR with a SNOMED CT code Behcet's disease). 75/227 (33%) EHR passed our internal review. Thirty-six reports were returned to the GP. Feedback was available for 28/36 of the reports sent. GP categorised nine reports as "Reasonable possible diagnosis" (advance for investigation), six reports as "diagnosis has already been excluded", ten reports as "patient has a clear alternative aetiology", and three reports as "Other" (patient left study locality, unable to re-identify accurately). All the 9 cases considered as "reasonable possible diagnosis" had further evaluation.
Conclusions: This pilot demonstrates that implementing such a tool is feasible at a population level. The case-finding tool identified credible cases which were subsequently referred for further investigation. Future work includes performance-based validation studies of diagnostic algorithms and the scalability of the tool.
Keywords: Database analysis; Electronic health records; Primary care; Rare disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Somanadhan S, Nicholson E, Dorris E, Brinkley A, Kennan A, Treacy E, Atif A, Ennis S, McGrath V, Mitchell D, O'Sullivan G, Power J, Lawlor A, Harkin P, Lynch SA, Watt P, Daly A, Donnelly S, Kroll T. Rare disease research partnership (RAinDRoP): a collaborative approach to identify research priorities for rare diseases in Ireland. HRB Open Res. 2020;11(3):13. doi: 10.12688/hrbopenres.13017.2.PMID:33299965;PMCID:PMC7702160. - DOI - PMC - PubMed
-
- Richter T, Nestler-Parr S, Babela R, Khan ZM, Tesoro T, Molsen E, Hughes DA, International Society for Pharmacoeconomics and Outcomes Research Rare Disease Special Interest Group. Rare disease terminology and definitions—a systematic global review: report of the ISPOR rare disease special interest group. Value Health. 2015;18(6):906–14. 10.1016/j.jval.2015.05.008. - PubMed
-
- The UK Rare Diseases Framework [Internet]. GOV.UK. 2021 [cited 2021 May 18]. https://www.gov.uk/government/publications/uk-rare-diseases-framework/th....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous