OxVent: Design and evaluation of a rapidly-manufactured Covid-19 ventilator
- PMID: 35172957
- PMCID: PMC8842095
- DOI: 10.1016/j.ebiom.2022.103868
OxVent: Design and evaluation of a rapidly-manufactured Covid-19 ventilator
Abstract
Background: The manufacturing of any standard mechanical ventilator cannot rapidly be upscaled to several thousand units per week, largely due to supply chain limitations. The aim of this study was to design, verify and perform a pre-clinical evaluation of a mechanical ventilator based on components not required for standard ventilators, and that met the specifications provided by the Medicines and Healthcare Products Regulatory Agency (MHRA) for rapidly-manufactured ventilator systems (RMVS).
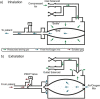
Methods: The design utilises closed-loop negative feedback control, with real-time monitoring and alarms. Using a standard test lung, we determined the difference between delivered and target tidal volume (VT) at respiratory rates between 20 and 29 breaths per minute, and the ventilator's ability to deliver consistent VT during continuous operation for >14 days (RMVS specification). Additionally, four anaesthetised domestic pigs (3 male-1 female) were studied before and after lung injury to provide evidence of the ventilator's functionality, and ability to support spontaneous breathing.
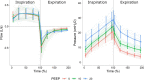
Findings: Continuous operation lasted 23 days, when the greatest difference between delivered and target VT was 10% at inspiratory flow rates >825 mL/s. In the pre-clinical evaluation, the VT difference was -1 (-90 to 88) mL [mean (LoA)], and positive end-expiratory pressure (PEEP) difference was -2 (-8 to 4) cmH2O. VT delivery being triggered by pressures below PEEP demonstrated spontaneous ventilation support.
Interpretation: The mechanical ventilator presented meets the MHRA therapy standards for RMVS and, being based on largely available components, can be manufactured at scale.
Funding: Work supported by Wellcome/EPSRC Centre for Medical Engineering,King's Together Fund and Oxford University.
Keywords: Biomedical engineering; Covid-19; Critical care; Respiration (artificial).
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests FFo reports grants from the National Institute for Health Research (UK), the National Institute of Academic Anaesthesia, and the Wellcome/EPSRC Centre for Medical Engineering. AF, FFo, SO and MT are volunteering directors of OxVent, a joint-venture social enterprise for mechanical ventilation between Oxford University and King's College London. TD is on the advisory board of OxVent. AAC-P, AF, FFo, MT, PG, SO and TD have shares in OxVent Ltd. AH and CVF are part-time employees of OxVent Ltd.
Figures






References
-
- Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329. - PubMed
-
- Medicines and Healthcare products Regulatory Agency. Specification for ventilators to be used in UK hospitals during the coronavirus (COVID-19) outbreak - GOV.UK [Internet]. 2020 [cited 2021 Sep 24]. Available from: https://www.gov.uk/government/publications/specification-for-ventilators...
-
- US Food and Drug Administration. Ventilators and ventilator accessories emergency use authorizations [Internet]. 2021 [cited 2021 Sep 24]. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em...
-
- Comisión Federal para la Protección contra Riesgos Sanitarios. Disposiciones para la adquisición y fabricación de ventiladores, durante la emergencia de salud pública por coronavirus 2019 (COVID-19) [Internet]. 2020 [cited 2021 Sep 24]. Available from: https://www.gob.mx/cms/uploads/attachment/file/545341/Disposiciones_para...
-
- Linklaters L.L.P. International Trade Committee inquiry: the COVID-19 pandemic and international trade. 2020.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

