PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma
- PMID: 35173040
- PMCID: PMC9664131
- DOI: 10.1136/gutjnl-2021-326350
PD-L1 blockade liberates intrinsic antitumourigenic properties of glycolytic macrophages in hepatocellular carcinoma
Abstract
Objective: Patients with increased PD-L1+ host cells in tumours are more potent to benefit from antiprogrammed death-1/programmed death ligand-1 (PD-L1) treatment, but the underlying mechanism is still unclear. We aim to elucidate the nature, regulation and functional relevance of PD-L1+ host cells in hepatocellular carcinoma (HCC).
Design: A total of untreated 184 HCC patients was enrolled randomly. C57BL/6 mice are given injection of Hepa1-6 cells to form autologous hepatoma. ELISpot, flow cytometry and real-time PCR are applied to analyse the phenotypic characteristics of PD-L1+ cells isolated directly from HCC specimens paired with blood samples or generated from ex vivo and in vitro culture systems. Immunofluorescence and immunohistochemistry are performed to detect the presence of immune cells on paraffin-embedded and formalin-fixed samples. The underlying regulatory mechanisms of metabolic switching are assessed by both in vitro and in vivo studies.
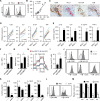
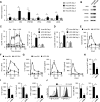
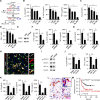
Results: We demonstrate that PD-L1+ host macrophages, which constructively represent the major cellular source of PD-L1 in HCC tumours, display an HLA-DRhighCD86high glycolytic phenotype, significantly produce antitumourigenic IL-12p70 and are polarised by intrinsic glycolytic metabolism. Mechanistically, a key glycolytic enzyme PKM2 triggered by hepatoma cell derived fibronectin 1, via a HIF-1α-dependent manner, concurrently controls the antitumourigenic properties and inflammation-mediated PD-L1 expression in glycolytic macrophages. Importantly, although increased PKM2+ glycolytic macrophages predict poor prognosis of patients, blocking PD-L1 on these cells eliminates PD-L1-dominant immunosuppression and liberates intrinsic antitumourigenic properties.
Conclusions: Selectively modulating the 'context' of glycolytic macrophages in HCC tumours might restore their antitumourigenic properties and provide a precise strategy for anticancer therapy.
Keywords: cancer immunobiology; glucose metabolism; hepatoma; immunotherapy; macrophages.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
