Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction
- PMID: 35173051
- PMCID: PMC8872724
- DOI: 10.1073/pnas.2115139119
Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction
Abstract
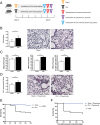
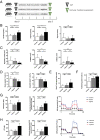
Severe sepsis induces a sustained immune dysfunction associated with poor clinical behavior. In particular, lymphopenia along with increased lymphocyte apoptosis and decreased lymphocyte proliferation, enhanced circulating regulatory T cells (Treg), and the emergence of myeloid-derived suppressor cells (MDSCs) have all been associated with persistent organ dysfunction, secondary infections, and late mortality. The mechanisms involved in MDSC-mediated T cell dysfunction during sepsis share some features with those described in malignancies such as arginine deprivation. We hypothesized that increasing arginine availability would restore T cell function and decrease sepsis-induced immunosuppression. Using a mouse model of sepsis based on cecal ligation and puncture and secondary pneumonia triggered by methicillin-resistant Staphylococcus aureus inoculation, we demonstrated that citrulline administration was more efficient than arginine in increasing arginine plasma levels and restoring T cell mitochondrial function and proliferation while reducing sepsis-induced Treg and MDSC expansion. Because there is no specific therapeutic strategy to restore immune function after sepsis, we believe that our study provides evidence for developing citrulline-based clinical studies in sepsis.
Keywords: T cell; citrulline; mitochondria; sepsis.
Conflict of interest statement
The authors declare no competing interest.
Figures


Comment in
-
Dietary modification of myeloid-derived suppressor cells (MDSC) activity in sepsis.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2201396119. doi: 10.1073/pnas.2201396119. Epub 2022 Mar 15. Proc Natl Acad Sci U S A. 2022. PMID: 35290112 Free PMC article. No abstract available.
References
-
- Angus D. C., van der Poll T., Severe sepsis and septic shock. N. Engl. J. Med. 369, 840–851 (2013). - PubMed
-
- Benjamim C. F., Hogaboam C. M., Kunkel S. L., The chronic consequences of severe sepsis. J. Leukoc. Biol. 75, 408–412 (2004). - PubMed
-
- Venet F., Monneret G., Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 14, 121–137 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

