Population antibody responses following COVID-19 vaccination in 212,102 individuals
- PMID: 35173150
- PMCID: PMC8850615
- DOI: 10.1038/s41467-022-28527-x
Population antibody responses following COVID-19 vaccination in 212,102 individuals
Abstract
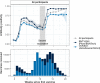
Population antibody surveillance helps track immune responses to COVID-19 vaccinations at scale, and identify host factors that may affect antibody production. We analyse data from 212,102 vaccinated individuals within the REACT-2 programme in England, which uses self-administered lateral flow antibody tests in sequential cross-sectional community samples; 71,923 (33.9%) received at least one dose of BNT162b2 vaccine and 139,067 (65.6%) received ChAdOx1. For both vaccines, antibody positivity peaks 4-5 weeks after first dose and then declines. At least 21 days after second dose of BNT162b2, close to 100% of respondents test positive, while for ChAdOx1, this is significantly reduced, particularly in the oldest age groups (72.7% [70.9-74.4] at ages 75 years and above). For both vaccines, antibody positivity decreases with age, and is higher in females and those with previous infection. Antibody positivity is lower in transplant recipients, obese individuals, smokers and those with specific comorbidities. These groups will benefit from additional vaccine doses.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures

References
-
- Lopez Bernal, J. et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N. Engl. J. Med. 10.1056/NEJMoa2108891 (2021). - PubMed
-
- Ward, H. et al. REACT-2 Round 5: increasing prevalence of SARS-CoV-2 antibodies demonstrate impact of the second wave and of vaccine roll-out in England. medRxiv10.1101/2021.02.26.21252512 (2021).
Publication types
MeSH terms
Substances
Grants and funding
- MC_PC_19012/MRC_/Medical Research Council/United Kingdom
- 200861/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- UNS32973/WT_/Wellcome Trust/United Kingdom
- MR/J008761/1/MRC_/Medical Research Council/United Kingdom
- MR/S019669/1/MRC_/Medical Research Council/United Kingdom
- RE/18/4/34215/BHF_/British Heart Foundation/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
- CC/CDC HHS/United States
- MR/L01341X/1/MRC_/Medical Research Council/United Kingdom
- MR/W029200/1/MRC_/Medical Research Council/United Kingdom
- 200187/Z/15/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

