Panoramix SUMOylation on chromatin connects the piRNA pathway to the cellular heterochromatin machinery
- PMID: 35173350
- PMCID: PMC11749891
- DOI: 10.1038/s41594-022-00721-x
Panoramix SUMOylation on chromatin connects the piRNA pathway to the cellular heterochromatin machinery
Abstract
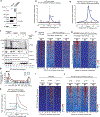
Nuclear Argonaute proteins, guided by small RNAs, mediate sequence-specific heterochromatin formation. The molecular principles that link Argonaute-small RNA complexes to cellular heterochromatin effectors on binding to nascent target RNAs are poorly understood. Here, we explain the mechanism by which the PIWI-interacting RNA (piRNA) pathway connects to the heterochromatin machinery in Drosophila. We find that Panoramix, a corepressor required for piRNA-guided heterochromatin formation, is SUMOylated on chromatin in a Piwi-dependent manner. SUMOylation, together with an amphipathic LxxLL motif in Panoramix's intrinsically disordered repressor domain, are necessary and sufficient to recruit Small ovary (Sov), a multi-zinc-finger protein essential for general heterochromatin formation and viability. Structure-guided mutations that eliminate the Panoramix-Sov interaction or that prevent SUMOylation of Panoramix uncouple Sov from the piRNA pathway, resulting in viable but sterile flies in which Piwi-targeted transposons are derepressed. Thus, Piwi engages the heterochromatin machinery specifically at transposon loci by coupling recruitment of a corepressor to nascent transcripts with its SUMOylation.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



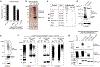


References
-
- Fedoroff NV Presidential address. Transposable elements, epigenetics, and genome evolution. Science 338, 758–67 (2012). - PubMed
-
- Janssen A, Colmenares SU & Karpen GH Heterochromatin: Guardian of the Genome. Annu Rev Cell Dev Biol 34, 265–288 (2018). - PubMed
-
- Grewal SI & Moazed D Heterochromatin and epigenetic control of gene expression. Science 301,798–802(2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

