Exploring the role of crystal habit in the Ostwald rule of stages
- PMID: 35173519
- PMCID: PMC8826364
- DOI: 10.1098/rspa.2021.0601
Exploring the role of crystal habit in the Ostwald rule of stages
Abstract
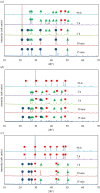
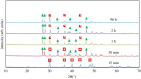
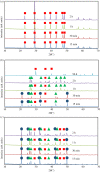
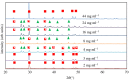
The crystallization of calcium carbonate is shown to be dictated by the Ostwald rule of stages (ORS), for high relative initial supersaturations ( ), under sweet (carbon dioxide saturated) and anoxic (oxygen depleted) solution conditions. Rhombohedral calcite crystals emerge after the sequential crystallization and dissolution of the metastable polymorphs: vaterite (snowflake-shaped) and aragonite (needle-shaped). However, the presence of certain cations, which can form trigonal carbonates (e.g. Fe2+ and Ni2+), in concentrations as low as 1.5 mM, triggers the emergence of calcite crystals, with a star-shaped crystal habit, first. These star-shaped crystals dissolve to yield needle-shaped aragonite crystals, which in turn dissolve to give the rhombohedral calcite crystals. The star-shaped crystals, formed at high SCaCO3 , possess higher surface free energy (therefore higher apparent solubility) than their rhombohedral counterparts. This sequence of dissolution and recrystallization demonstrates that the ORS does not only drive the crystal towards its thermodynamically most stable polymorph but also towards its most stable crystal habit.
Keywords: Ostwald rule of stages; calcium carbonate; crystal habit; polymorphism; surface energy.
© 2022 The Authors.
Figures





References
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
