Longitudinal Trajectories of Participant- and Study Partner-Rated Cognitive Decline, in Relation to Alzheimer's Disease Biomarkers and Mood Symptoms
- PMID: 35173601
- PMCID: PMC8841868
- DOI: 10.3389/fnagi.2021.806432
Longitudinal Trajectories of Participant- and Study Partner-Rated Cognitive Decline, in Relation to Alzheimer's Disease Biomarkers and Mood Symptoms
Abstract
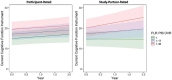
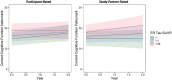
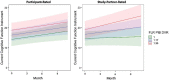
Whereas discrepancies between participant- and study partner-reported cognitive concerns on the Alzheimer's disease (AD) continuum have been observed, more needs to be known regarding the longitudinal trajectories of participant- vs. study partner-reported concerns, particularly their relationship to AD biomarkers and mood symptomology. Additionally, it is unclear whether years of in-clinic data collection are needed to observe relationships with AD biomarkers, or whether more frequent, remote assessments over shorter periods of time would suffice. This study primarily sought to examine the relationships between longitudinal trajectories of participant- and study partner-rated cognitive decline and baseline biomarker levels [i.e., amyloid and tau positron emission tomography (PET)], in addition to how mood symptomatology may alter these trajectories of concerns over a 2-year period. Baseline mood was associated with longitudinal participant-rated concerns, such that participants with elevated depression and anxiety scores at baseline had decreasing concerns about cognitive decline over time (fixed estimate = -0.17, 95% CI [-0.29 to -0.05], t = -2.75, df = 457, adj. p = 0.012). A significant interaction between baseline amyloid (fixed estimate = 4.07, 95% CI [1.13-7.01], t = 2.72, df = 353, adj. p = 0.026) and tau (fixed estimate = 3.50, 95% CI [0.95-6.06], t = 2.70, df = 331, adj. p = 0.030) levels was associated with increasing study partner concerns, but not participant concerns, over time. The interaction between amyloid and study partner concerns remained significant when utilizing only the first year of concern-related data collection. Overall, these results suggest that frequent, remote assessment of study partner-reported concerns may offer additional insight into the AD clinical spectrum, as study partners appear to more accurately update their concerns over time with regard to pathology, with these concerns less influenced by participants' mood symptomatology.
Keywords: Alzheimer’s disease; amyloid; anxiety; cognitive concerns; depression; longitudinal; mood; tau.
Copyright © 2022 Munro, Buckley, Vannini, DeMuro, Sperling, Rentz, Johnson, Gatchel and Amariglio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Relation of modifiable lifestyle and mood factors to cognitive concerns among participants and their study partners in the A4 screen data.Alzheimers Dement (Amst). 2023 Jun 7;15(2):e12435. doi: 10.1002/dad2.12435. eCollection 2023 Apr-Jun. Alzheimers Dement (Amst). 2023. PMID: 37304049 Free PMC article.
-
Plasma Aβ42/40 ratio, p-tau181, GFAP, and NfL across the Alzheimer's disease continuum: A cross-sectional and longitudinal study in the AIBL cohort.Alzheimers Dement. 2023 Apr;19(4):1117-1134. doi: 10.1002/alz.12724. Epub 2022 Jul 21. Alzheimers Dement. 2023. PMID: 36574591
-
Item-Level Investigation of Participant and Study Partner Report on the Cognitive Function Index from the A4 Study Screening Data.J Prev Alzheimers Dis. 2021;8(3):257-262. doi: 10.14283/jpad.2021.8. J Prev Alzheimers Dis. 2021. PMID: 34101781 Free PMC article.
-
2014 Update of the Alzheimer's Disease Neuroimaging Initiative: A review of papers published since its inception.Alzheimers Dement. 2015 Jun;11(6):e1-120. doi: 10.1016/j.jalz.2014.11.001. Alzheimers Dement. 2015. PMID: 26073027 Free PMC article. Review.
-
The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception.Alzheimers Dement. 2013 Sep;9(5):e111-94. doi: 10.1016/j.jalz.2013.05.1769. Epub 2013 Aug 7. Alzheimers Dement. 2013. PMID: 23932184 Free PMC article. Review.
Cited by
-
Gender of Study Partners and Research Participants Associated With Differences in Study Partner Ratings of Cognition and Activity Level.J Gerontol B Psychol Sci Soc Sci. 2023 Aug 2;78(8):1318-1329. doi: 10.1093/geronb/gbad026. J Gerontol B Psychol Sci Soc Sci. 2023. PMID: 36790294 Free PMC article.
-
Study Partner Report of Apathy in Older Adults is Associated with AD Biomarkers: Findings from the Harvard Aging Brain Study.Am J Geriatr Psychiatry. 2024 Aug;32(8):909-919. doi: 10.1016/j.jagp.2024.01.020. Epub 2024 Jan 28. Am J Geriatr Psychiatry. 2024. PMID: 38443298 Free PMC article.
-
Genetic Risk for Alzheimer's Disease Alters Perceived Executive Dysfunction in Cognitively Healthy Middle-Aged and Older Adults.J Alzheimers Dis Rep. 2024 Feb 16;8(1):267-279. doi: 10.3233/ADR-230166. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 38405345 Free PMC article.
-
Everyday Functioning and Entorhinal and Inferior Temporal Tau Burden in Cognitively Normal Older Adults.J Prev Alzheimers Dis. 2022;9(4):801-808. doi: 10.14283/jpad.2022.58. J Prev Alzheimers Dis. 2022. PMID: 36281685 Free PMC article.
-
Predicting progression of cognitive decline to dementia using dyadic patterns of subjective reporting: evidence from the CompAS longitudinal study.Front Aging Neurosci. 2024 Feb 2;16:1319743. doi: 10.3389/fnagi.2024.1319743. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38371398 Free PMC article.
References
-
- Benjamini Y., Hochburg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57 289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

