The Effects of Acute and Chronic Selective Phosphodiesterase 1 Inhibition on Smooth Muscle Cell-Associated Aging Features
- PMID: 35173613
- PMCID: PMC8841451
- DOI: 10.3389/fphar.2021.818355
The Effects of Acute and Chronic Selective Phosphodiesterase 1 Inhibition on Smooth Muscle Cell-Associated Aging Features
Abstract
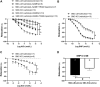
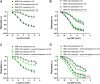
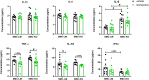
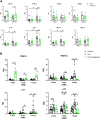
Age-related cardiovascular diseases (CVDs) remain among the leading global causes of death, and vascular smooth muscle cell (VSMC) remodeling plays an essential role in its pathology. Reduced NO-cGMP pathway signaling is a major feature and pathogenic mechanism underlying vasodilator dysfunction. Recently, we identified phosphodiesterase (PDE) 1, an enzyme that hydrolyzes and inactivates the cyclic nucleotides cAMP and cGMP, and thereby provides a potential treatment target for restoring age-related vascular dysfunction due to aging of VSMC. Based on this hypothesis, we here tested the effects of PDE1 inhibition in a model of SMC-specific accelerated aging mice. SMC-KO and their WT littermates received either vehicle or the PDE1 inhibitor lenrispodun for 8 weeks. Vascular function was measured both in vivo (Laser Doppler technique) and ex vivo (organ bath). Moreover, we deployed UV irradiation in cell culture experiments to model accelerated aging in an in vitro situation. SMC-KO mice display a pronounced loss of vasodilator function in the isolated aorta, the cutaneous microvasculature, and mesenteric arteries. Ex vivo, in isolated vascular tissue, we found that PDE1 inhibition with lenrispodun improves vasodilation, while no improvement was observed in isolated aorta taken from mice after chronic treatment in vivo. However, during lenrispodun treatment in vivo, an enhanced microvascular response in association with upregulated cGMP levels was seen. Further, chronic lenrispodun treatment decreased TNF-α and IL-10 plasma levels while the elevated level of IL-6 in SMC-KO mice remained unchanged after treatment. PDE1 and senescence markers, p16 and p21, were increased in both SMC-KO aorta and cultured human VSMC in which DNA was damaged by ultraviolet irradiation. This increase was lowered by chronic lenrispodun. In contrast, lenrispodun increased the level of PDE1A in both situations. In conclusion, we demonstrated that PDE1 inhibition may be therapeutically useful in reversing aspects of age-related VSMC dysfunction by potentiating NO-cGMP signaling, preserving microvascular function, and decreasing senescence. Yet, after chronic treatment, the effects of PDE1 inhibition might be counteracted by the interplay between differential PDE1A and C expression. These results warrant further pharmacodynamic profiling of PDE enzyme regulation during chronic PDE1 inhibitor treatment.
Keywords: NO-cGMP pathway; cardiovascular; inflammation; phosphodiesterase; smooth muscle cell; vascular aging.
Copyright © 2022 Golshiri, Ataabadi, Jüttner, Snyder, Davis, Lin, Zhang, de Vries, Garrelds, Leijten, Danser and Roks.
Conflict of interest statement
GS, RD, AL, and LZ were employed by Intracellular-Therapies, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Phosphodiesterase 1 regulation is a key mechanism in vascular aging.Clin Sci (Lond). 2015 Dec;129(12):1061-75. doi: 10.1042/CS20140753. Epub 2015 Jun 25. Clin Sci (Lond). 2015. PMID: 26464516
-
Vascular Ageing Features Caused by Selective DNA Damage in Smooth Muscle Cell.Oxid Med Cell Longev. 2021 Aug 31;2021:2308317. doi: 10.1155/2021/2308317. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34504640 Free PMC article.
-
PDE1A inhibition elicits cGMP-dependent relaxation of rat mesenteric arteries.Br J Pharmacol. 2017 Nov;174(22):4186-4198. doi: 10.1111/bph.14034. Epub 2017 Oct 15. Br J Pharmacol. 2017. PMID: 28910498 Free PMC article.
-
Phosphodiesterase 1: A Unique Drug Target for Degenerative Diseases and Cognitive Dysfunction.Adv Neurobiol. 2017;17:349-384. doi: 10.1007/978-3-319-58811-7_13. Adv Neurobiol. 2017. PMID: 28956339 Review.
-
Cyclic GMP phosphodiesterases and regulation of smooth muscle function.Circ Res. 2003 Aug 22;93(4):280-91. doi: 10.1161/01.RES.0000087541.15600.2B. Circ Res. 2003. PMID: 12933699 Review.
Cited by
-
Soluble guanylate cyclase activator BAY 54-6544 improves vasomotor function and survival in an accelerated ageing mouse model.Aging Cell. 2022 Sep;21(9):e13683. doi: 10.1111/acel.13683. Epub 2022 Aug 27. Aging Cell. 2022. PMID: 36029161 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

