Total Glucosides of Paeonia lactiflora for Safely Reducing Disease Activity in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis
- PMID: 35173622
- PMCID: PMC8841895
- DOI: 10.3389/fphar.2022.834947
Total Glucosides of Paeonia lactiflora for Safely Reducing Disease Activity in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis
Abstract
Background: Total glucosides of paeony (TGP), extracted from the dried roots of Paeonia lactiflora Pall., are proven to regulate immune function in various rheumatic diseases. We aim to systematically evaluate the efficacy and safety of TGP in reducing disease activity in systemic lupus erythematosus (SLE). Methods: We searched trials in seven electronic databases and two clinical trail registries. Randomized controlled trials (RCTs) evaluating efficacy and safety of TGP for SLE were identified. The Cochrane Risk of Bias Tool 2.0 was used for quality assessment of the included trials, and RevMan 5.4 software was used for meta-analysis. Results: A total of 14 RCTs were included, including 978 participants, 492 in the intervention group and 486 in the control group. Regarding the efficacy of TGP for SLE, results showed that TGP plus conventional treatments (CTs) was superior to CTs alone in reducing disease activity (MD SLEDAI-1m = -3.54, 95% CI = -4.08 to -3.00, p < 0.00001; MD SLEDAI-2m = -3.80, 95% CI = -4.51 to -3.09, p < 0.00001; MD SLEDAI-3m = -1.62, 95% CI = -2.60 to -0.64, p < 0.0001; MD SLEDAI-6m = -1.97, 95% CI = -3.18 to -0.76, p = 0.001). The results also showed that TGP contributed to a betterment in improving other outcomes related to lupus activity, such as ESR, CRP, complement proteins (C3, C4), and immunoglobulins (IgA, IgM). In addition, TGP significantly decreased average daily glucocorticoid dosage and cumulative cyclophosamide dosage, as well as disease recurrence rate. In terms of safety, TGP may reduce the incidence of adverse reactions (RR = 0.51, 95% CI = 0.29 to 0.88, p = 0.01). The certainty of the evidence were assessed as moderate to low. Conclusion: TGP appears potentially effective and generally safe in reducing disease activity in SLE. However, in view of high risk of bias, the findings need to be confirmed in high-quality trials. Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD42021274850.
Keywords: disease activity; meta-analysis; safety; systemic lupus erythematosus; total glucosides of paeony.
Copyright © 2022 Chen, Wang, Cao and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
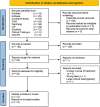








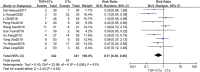

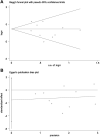
References
-
- Cai H. Y., Cai Z. H., Shao L. P. (2017). Therapeutic Effect of Total Glucosides of Paeony on Systemic Lupus Erythematosus. J. Chin. Physician 19 (03), 445–447. 10.3760/cma.j.issn.1008-1372.2017.03.036 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

