Evasion of Host Antiviral Innate Immunity by Paramyxovirus Accessory Proteins
- PMID: 35173691
- PMCID: PMC8841848
- DOI: 10.3389/fmicb.2021.790191
Evasion of Host Antiviral Innate Immunity by Paramyxovirus Accessory Proteins
Abstract
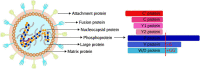
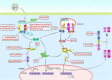
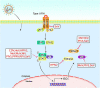
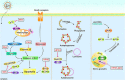
For efficient replication, viruses have developed multiple strategies to evade host antiviral innate immunity. Paramyxoviruses are a large family of enveloped RNA viruses that comprises diverse human and animal pathogens which jeopardize global public health and the economy. The accessory proteins expressed from the P gene by RNA editing or overlapping open reading frames (ORFs) are major viral immune evasion factors antagonizing type I interferon (IFN-I) production and other antiviral innate immune responses. However, the antagonistic mechanisms against antiviral innate immunity by accessory proteins differ among viruses. Here, we summarize the current understandings of immune evasion mechanisms by paramyxovirus accessory proteins, specifically how accessory proteins directly or indirectly target the adaptors in the antiviral innate immune signaling pathway to facilitate virus replication. Additionally, some cellular responses, which are also involved in viral replication, will be briefly summarized.
Keywords: IFN; accessory proteins; antiviral innate immunity; immune evasion; paramyxoviruses.
Copyright © 2022 Wang, Wang, Duan, Chen, Hu, Wang, Jia, Chu, Liu, Wang, Zhang, Xiao, Wang, Dang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Andrejeva J., Childs K. S., Young D. F., Carlos T. S., Stock N., Goodbourn S., et al. (2004). The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN- promoter. Proc. Natl. Acad. Sci. U.S.A. 101 17264–17269. 10.1073/pnas.0407639101 - DOI - PMC - PubMed
-
- Andrejeva J., Poole E., Young D. F., Goodbourn S., Randall R. E. (2002a). The p127 subunit (DDB1) of the UV-DNA damage repair binding protein is essential for the targeted degradation of STAT1 by the V protein of the paramyxovirus simian virus 5. J. Virol. 76 11379–11386. 10.1128/JVI.76.22.11379-11386.2002 - DOI - PMC - PubMed
-
- Andrejeva J., Young D. F., Goodbourn S., Randall R. E. (2002b). Degradation of STAT1 and STAT2 by the V proteins of Simian virus 5 and human parainfluenza virus type 2, respectively: consequences for virus replication in the presence of Alpha/Beta and gamma interferons. J. Virol. 76 2159–2167. 10.1128/jvi.76.5.2159-2167.2002 - DOI - PMC - PubMed
-
- Audsley M. D., Marsh G. A., Lieu K. G., Tachedjian M., Joubert D. A., Wang L.-F., et al. (2016). The immune evasion function of J and Beilong virus V proteins is distinct from that of other paramyxoviruses, consistent with their inclusion in the proposed genus Jeilongvirus. J. Virol. 97 581–592. 10.1099/jgv.0.000388 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

