Single-Cell Technologies for the Study of Antibody-Secreting Cells
- PMID: 35173713
- PMCID: PMC8841722
- DOI: 10.3389/fimmu.2021.821729
Single-Cell Technologies for the Study of Antibody-Secreting Cells
Abstract
Antibody-secreting cells (ASC), plasmablasts and plasma cells, are terminally differentiated B cells responsible for large-scale production and secretion of antibodies. ASC are derived from activated B cells, which may differentiate extrafollicularly or form germinal center (GC) reactions within secondary lymphoid organs. ASC therefore consist of short-lived, poorly matured plasmablasts that generally secrete lower-affinity antibodies, or long-lived, highly matured plasma cells that generally secrete higher-affinity antibodies. The ASC population is responsible for producing an immediate humoral B cell response, the polyclonal antibody repertoire, as well as in parallel building effective humoral memory and immunity, or potentially driving pathology in the case of autoimmunity. ASC are phenotypically and transcriptionally distinct from other B cells and further distinguishable by morphology, varied lifespans, and anatomical localization. Single cell analyses are required to interrogate the functional and transcriptional diversity of ASC and their secreted antibody repertoire and understand the contribution of individual ASC responses to the polyclonal humoral response. Here we summarize the current and emerging functional and molecular techniques for high-throughput characterization of ASC with single cell resolution, including flow and mass cytometry, spot-based and microfluidic-based assays, focusing on functional approaches of the secreted antibodies: specificity, affinity, and secretion rate.
Keywords: B cells; antibodies; antibody secreting cell; droplet microfluidics; functional bioassay; high-throuput technique; plasma cell (PC).
Copyright © 2022 Broketa and Bruhns.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. PB is a paid consultant to Regeneron Pharmaceuticals. MB declares that he has no relevant conflicts of interest.
Figures
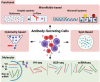
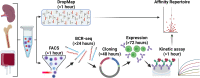
Similar articles
-
Protective Humoral Immunity in the Central Nervous System Requires Peripheral CD19-Dependent Germinal Center Formation following Coronavirus Encephalomyelitis.J Virol. 2017 Nov 14;91(23):e01352-17. doi: 10.1128/JVI.01352-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931676 Free PMC article.
-
Plasma cells: The programming of an antibody-secreting machine.Eur J Immunol. 2019 Jan;49(1):30-37. doi: 10.1002/eji.201847517. Epub 2018 Oct 15. Eur J Immunol. 2019. PMID: 30273443 Review.
-
An asymmetric antibody repertoire is shaped between plasmablasts and plasma cells after secondary immunization with (4-hydroxy-3-nitrophenyl)acetyl chicken γ-globulin.Int Immunol. 2015 Dec;27(12):609-20. doi: 10.1093/intimm/dxv040. Epub 2015 Jul 7. Int Immunol. 2015. PMID: 26152273
-
High-throughput single-cell screening of viable hybridomas and patient-derived antibody-secreting cells using punchable microwells.Artif Cells Nanomed Biotechnol. 2024 Dec;52(1):426-436. doi: 10.1080/21691401.2024.2395815. Epub 2024 Aug 29. Artif Cells Nanomed Biotechnol. 2024. PMID: 39206935
-
Plasma cell differentiation during the germinal center reaction.Immunol Rev. 2019 Mar;288(1):64-74. doi: 10.1111/imr.12751. Immunol Rev. 2019. PMID: 30874351 Review.
Cited by
-
Integrated Single-Cell (Phospho-)Protein and RNA Detection Uncovers Phenotypic Characteristics and Active Signal Transduction of Human Antibody-Secreting Cells.Mol Cell Proteomics. 2023 Feb;22(2):100492. doi: 10.1016/j.mcpro.2023.100492. Epub 2023 Jan 7. Mol Cell Proteomics. 2023. PMID: 36623694 Free PMC article.
-
TRAPnSeq allows high-throughput profiling of antigen-specific antibody-secreting cells.Cell Rep Methods. 2023 Jul 7;3(7):100522. doi: 10.1016/j.crmeth.2023.100522. eCollection 2023 Jul 24. Cell Rep Methods. 2023. PMID: 37533642 Free PMC article.
-
Intranasal Inoculation of Cationic Crosslinked Carbon Dots-Adjuvanted Respiratory Syncytial Virus F Subunit Vaccine Elicits Mucosal and Systemic Humoral and Cellular Immunity.MedComm (2020). 2025 Mar 24;6(4):e70146. doi: 10.1002/mco2.70146. eCollection 2025 Apr. MedComm (2020). 2025. PMID: 40135196 Free PMC article.
-
Human lymphoid tissue sampling for vaccinology.Front Immunol. 2022 Nov 17;13:1045529. doi: 10.3389/fimmu.2022.1045529. eCollection 2022. Front Immunol. 2022. PMID: 36466924 Free PMC article. Review.
-
Qualitative monitoring of SARS-CoV-2 mRNA vaccination in humans using droplet microfluidics.JCI Insight. 2023 Jul 10;8(13):e166602. doi: 10.1172/jci.insight.166602. JCI Insight. 2023. PMID: 37252802 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

