Inactivated Rabies Virus Vectored MERS-Coronavirus Vaccine Induces Protective Immunity in Mice, Camels, and Alpacas
- PMID: 35173733
- PMCID: PMC8842186
- DOI: 10.3389/fimmu.2022.823949
Inactivated Rabies Virus Vectored MERS-Coronavirus Vaccine Induces Protective Immunity in Mice, Camels, and Alpacas
Abstract
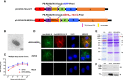
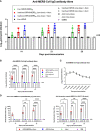
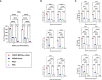
Middle East respiratory syndrome coronavirus (MERS-CoV) is an emergent coronavirus that has caused frequent zoonotic events through camel-to-human spillover. An effective camelid vaccination strategy is probably the best way to reduce human exposure risk. Here, we constructed and evaluated an inactivated rabies virus-vectored MERS-CoV vaccine in mice, camels, and alpacas. Potent antigen-specific antibody and CD8+ T-cell responses were generated in mice; moreover, the vaccination reduced viral replication and accelerated virus clearance in MERS-CoV-infected mice. Besides, protective antibody responses against both MERS-CoV and rabies virus were induced in camels and alpacas. Satisfyingly, the immune sera showed broad cross-neutralizing activity against the three main MERS-CoV clades. For further characterization of the antibody response induced in camelids, MERS-CoV-specific variable domains of heavy-chain-only antibody (VHHs) were isolated from immunized alpacas and showed potent prophylactic and therapeutic efficacies in the Ad5-hDPP4-transduced mouse model. These results highlight the inactivated rabies virus-vectored MERS-CoV vaccine as a promising camelid candidate vaccine.
Keywords: MERS-CoV; alpaca; camel; mice; rabies virus vector; vaccine.
Copyright © 2022 Chi, Wang, Li, Wang, Wang, Jin, Han, Wang, Wang, Zhu, Sun, Zhuang, Zhang, Ye, Wang, Feng, Hu, Gao, Zhao, Zhao, Yang and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Comment in
-
Commentary: Inactivated rabies virus vectored MERS-Coronavirus vaccine induces protective immunity in mice, camels, and alpacas.Front Immunol. 2025 Mar 4;16:1549481. doi: 10.3389/fimmu.2025.1549481. eCollection 2025. Front Immunol. 2025. PMID: 40103811 Free PMC article. No abstract available.
References
-
- World Health Organization . Disease Outbreak News (DONs) . Available at: https://www.who.int/emergencies/disease-outbreak-news.
-
- World Health Organization . WHO MERS Global Summary and Assessment of Risk . Available at: http://www.who.int/csr/disease/coronavirus_infections/risk-assessment-au....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

