Maternal Overnutrition During Gestation in Sheep Alters Autophagy Associated Pathways in Offspring Heart
- PMID: 35173761
- PMCID: PMC8841792
- DOI: 10.3389/fgene.2021.742704
Maternal Overnutrition During Gestation in Sheep Alters Autophagy Associated Pathways in Offspring Heart
Abstract
Poor maternal nutrition during gestation can negatively affect offspring growth, development, and health pre- and post-natally. Overfeeding during gestation or maternal obesity (MO) results in altered metabolism and imbalanced endocrine hormones in animals and humans which will have long-lasting and detrimental effects on offspring growth and health. In this study, we examined the effects of overnutrition during gestation on autophagy associated pathways in offspring heart muscles at two gestational and one early postnatal time point (n = 5 for treated and untreated male and female heart respectively at each time point). Two-way ANOVA was used to analyze the interaction between treatment and sex at each time point. Our results revealed significant interactions of maternal diet by developmental stages for offspring autophagy signaling. Overfeeding did not affect the autophagy signaling at mid-gestation day 90 (GD90) in both male and female offspring while the inflammatory cytokines were increased in GD90 MO male offsrping; however, overfeeding during gestation significantly increased autophagy signaling, but not inflammation level at a later developmental stage (GD135 and day 1 after birth) in both males and females. We also identified a sexual dimorphic response in which female progeny were more profoundly influenced by maternal diet than male progeny regardless of developmental stages. We also determined the cortisol concentrations in male and female hearts at three developmental stages. We did not observe cortisol changes between males and females or between overfeeding and control groups. Our exploratory studies imply that MO alters autophagy associated pathways in both male and female at later developmental stages with more profound effects in female. This finding need be confirmed with larger sample numbers in the future. Our results suggest that targeting on autophagy pathway could be a strategy for correction of adverse effects in offspring of over-fed ewes.
Keywords: autophagy; developmental programming; heart muscle; maternal obesity; poor maternal nutrition; sheep.
Copyright © 2022 Liu, Ding, Halderson, Arriola Apelo, Jones, Pillai, Hoffman, Reed, Govoni, Zinn and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



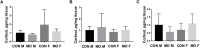


References
Grants and funding
LinkOut - more resources
Full Text Sources

