Risk of bias in non-randomized observational studies assessing the relationship between proton-pump inhibitors and adverse kidney outcomes: a systematic review
- PMID: 35173802
- PMCID: PMC8841917
- DOI: 10.1177/17562848221074183
Risk of bias in non-randomized observational studies assessing the relationship between proton-pump inhibitors and adverse kidney outcomes: a systematic review
Abstract
Background: Proton-pump inhibitors (PPIs) are widely prescribed as acid-suppression therapy. Some observational studies suggest that long-term use of PPIs is potentially associated with certain adverse kidney outcomes. We conducted a systematic literature review to assess potential bias in non-randomized studies reporting on putative associations between PPIs and adverse kidney outcomes (acute kidney injury, acute interstitial nephritis, chronic interstitial nephritis, acute tubular necrosis, chronic kidney disease, and end-stage renal disease).
Methods: We searched the medical literature within 10 years of 17 December 2020. Pre-specified criteria guided identification of relevant English language articles for assessment. Risk of bias on an outcome-specific basis was evaluated using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool by two independent reviewers.
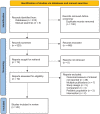
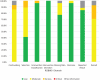
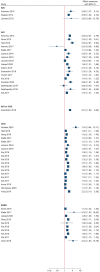
Results: Of 620 initially identified records, 26 studies met a priori eligibility criteria and underwent risk of bias assessment. Nineteen studies were judged as having a moderate risk of bias for reported adverse kidney outcomes, while six studies were judged as having a serious risk of bias (mainly due to inadequate control of confounders and selection bias). We were unable to determine the overall risk of bias in two studies (one of which was assessed as having a moderate risk of bias for a different adverse kidney outcome) due to insufficient information presented. Effect estimates for PPIs in relation to adverse kidney outcomes varied widely (0.24-7.34) but associations mostly showed increased risk.
Conclusion: Using ROBINS-I, we found that non-randomized observational studies suggesting kidney harm by PPIs have moderate to serious risk of bias, making it challenging to establish causality. Additional high-quality, real-world evidence among generalizable populations are needed to better understand the relation between PPI treatment and acute and chronic kidney outcomes, accounting for the effects of varying durations of PPI treatment, self-treatment with over-the-counter PPIs, and potential critical confounders.
Keywords: acute interstitial nephritis; acute kidney injury; acute tubular necrosis; chronic kidney disease; end-stage renal disease; proton-pump inhibitors.
© The Author(s), 2022.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Takeda Pharmaceuticals contracted with CERobs Consulting, LLC, a consulting firm with focus on real-world evidence observational research, comparative effectiveness research, pragmatic randomized trials, outcomes research, epidemiology, and clinical outcome assessments, including patient-reported outcomes. PR, KI, TR, and CJG consulted on this project through CERobs Consulting, LLC. KK-Z has received honoraria and/or support from Abbott, Abbvie, ACI Clinical (Cara Therapeutics), Akebia, Alexion, Amgen, Ardelyx, ASN (American Society of Nephrology), Astra-Zeneca, Aveo, BBraun, Chugai, Cytokinetics, Daiichi, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, IFKF (International Federation of Kidney Foundations), ISH (International Society of Hemodialysis), International Society of Renal Nutrition & Metabolism (ISRNM), JSDT (Japanese Society of Dialysis Therapy), Hospira, Kabi, Keryx, Kissei, Novartis, OPKO, NIH (National Institutes of Health), NKF (National Kidney Foundations), Pfizer, Regulus, Relypsa, Resverlogix, Dr Schaer, Sandoz, Sanofi, Shire, VA (Veterans’ Affairs), Takeda, Vifor, UpToDate, ZS-Pharma. DB is a salaried employee of Takeda Pharmaceuticals.
Figures
Similar articles
-
The association between proton pump inhibitor use and the risk of adverse kidney outcomes: a systematic review and meta-analysis.Nephrol Dial Transplant. 2018 Feb 1;33(2):331-342. doi: 10.1093/ndt/gfw470. Nephrol Dial Transplant. 2018. PMID: 28339835
-
Proton pump inhibitors for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2020 Aug 25;8(8):CD013113. doi: 10.1002/14651858.CD013113.pub2. Cochrane Database Syst Rev. 2020. PMID: 32844430 Free PMC article.
-
Recovery schools for improving behavioral and academic outcomes among students in recovery from substance use disorders: a systematic review.Campbell Syst Rev. 2018 Oct 4;14(1):1-86. doi: 10.4073/csr.2018.9. eCollection 2018. Campbell Syst Rev. 2018. PMID: 37131375 Free PMC article.
-
Proton Pump Inhibitors and the Kidney: Implications of Current Evidence for Clinical Practice and When and How to Deprescribe.Am J Kidney Dis. 2020 Apr;75(4):497-507. doi: 10.1053/j.ajkd.2019.07.012. Epub 2019 Oct 10. Am J Kidney Dis. 2020. PMID: 31606235 Review.
-
Proton-pump inhibitors and chronic kidney disease: Hidden consequences of an inappropriate drug use?Saudi J Kidney Dis Transpl. 2020 Mar-Apr;31(2):312-319. doi: 10.4103/1319-2442.284005. Saudi J Kidney Dis Transpl. 2020. PMID: 32394903 Review.
Cited by
-
Estimates of Chronic Kidney Diseases Associated with Proton-Pump Inhibitors Using a Retrospective Hospital-Based Cohort in Thailand.Int J Nephrol Renovasc Dis. 2022 Dec 10;15:371-381. doi: 10.2147/IJNRD.S389238. eCollection 2022. Int J Nephrol Renovasc Dis. 2022. PMID: 36530347 Free PMC article.
-
Repositioning of Lansoprazole as a Protective Agent Against Cisplatin-Induced Ototoxicity.Front Pharmacol. 2022 Jul 15;13:896760. doi: 10.3389/fphar.2022.896760. eCollection 2022. Front Pharmacol. 2022. PMID: 35910376 Free PMC article.
References
-
- Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology 2017; 153: 35–48. - PubMed
-
- Li T, Xie Y, Al-Aly Z. The association of proton pump inhibitors and chronic kidney disease: cause or confounding? Curr Opin Nephrol Hypertens 2018; 27: 182–187. - PubMed
-
- Mishuk A, Chen L, Gaillard P, et al.. National trends in prescription proton pump inhibitor use and expenditure in the United States in 2002-2017. J Am Pharm Assoc 2020; 61: 87–94. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

