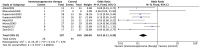Do early prednisolone and other immunosuppressant therapies prevent generalization in ocular myasthenia gravis in Western populations: a systematic review and meta-analysis
- PMID: 35173803
- PMCID: PMC8842340
- DOI: 10.1177/1756286419876521
Do early prednisolone and other immunosuppressant therapies prevent generalization in ocular myasthenia gravis in Western populations: a systematic review and meta-analysis
Abstract
Background: The majority of ocular myasthenia gravis (OMG) patients will progress to generalized myasthenia gravis (GMG), usually within 2 years of disease onset. The aim of this meta-analysis was to evaluate the effect of early prednisolone and other immunosuppressants therapy on the generalization rate in OMG patients.
Methods: We searched the CENTRAL, EMBASE, and MEDLINE databases via the Ovid SP database for all relevant publications on 16 July 2018.
Results: Eight studies comprising a total of 547 participants were included in our meta-analysis. Compared with pyridostigmine treatment, prednisolone and other immunosuppressants therapy produced an odds ratio (OR) for the development of GMG of 0.19 [95% confidence interval (CI), 0.11-0.30; I 2 = 37%], indicating that early prednisolone and other immunosuppressants therapy reduced the generalization rate in OMG by 81%.
Conclusions: Early prednisolone and other immunosuppressants therapy can significantly reduce the risk of generalization in OMG patients, and should be considered in newly diagnosed OMG patients. Due to the inclusion of retrospective studies, this noted effect might have been related to corticosteroids, especially when immunosuppressants used at low dosages and in mild disease. Additionally, the data derived from Western populations, thus a prospective randomized controlled trial (RCT) is warranted to confirm this effect of early prednisolone and other immunosuppressants therapy on OMG generalization both in Western and Asian populations.
Keywords: generalized myasthenia gravis; immunosuppressive therapy; meta-analysis; ocular myasthenia gravis; prednisolone; systematic review.
© The Author(s), 2019.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Benatar M, Kaminski HJ; Quality Standards Subcommittee of the American Academy of Neurology. Evidence report: the medical treatment of ocular myasthenia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2007; 68: 2144–2149. - PubMed
-
- Bever CT, Jr, Aquino AV, Penn AS, et al. Prognosis of ocular myasthenia. Ann Neurol 1983; 14: 516–519. - PubMed
-
- Kerty E, Elsais A, Argov Z, et al. EFNS/ENS Guidelines for the treatment of ocular myasthenia. EurJ Neurol 2014; 21: 687–693. - PubMed
-
- Jaretzki A, III, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 2000; 55: 16–23. - PubMed
LinkOut - more resources
Full Text Sources