Imaging findings of vinyl dimethyl polydimethylsiloxane used as a paraurethral injectable for female stress urinary incontinence
- PMID: 35173814
- PMCID: PMC8842146
- DOI: 10.1177/17562872211060909
Imaging findings of vinyl dimethyl polydimethylsiloxane used as a paraurethral injectable for female stress urinary incontinence
Abstract
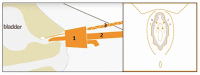
Objectives: Vinyl dimethyl polydimethylsiloxane (VDPDMS) is a urethral bulking agent used for female stress urinary incontinence (SUI), that is clearly visible on computed tomography (CT). Clinical effects are promising, but it remains difficult to identify factors predicting clinical success. Clinical outcome might depend on the shape and position of the implants after injection. Objective of this study is to analyze the appearance and position of bulk material on CT scans and to see whether it is delivered the intended circumferential and mid-urethral position.
Methods: A single-center retrospective study was performed in 20 women, treated with VDPDMS for SUI. A senior radiologist analyzed all CTs, using an assessment scheme. This scheme describes whether the bulk is scattered, mid-urethral, and/or circumferentially distributed. The imaging findings were subsequently correlated to the patient global impression of improvement (PGI-I) and the percentage of subjective improvement experienced 6 weeks post-operatively.
Results: The patient's mean age was 61 years, and they underwent median 2.0 previous surgical treatments for SUI. Three patients reported no improvement, 9 patients had 20-90% improvement and 8 reported >90% improvement of their SUI. In 17/74 (24%) positions, the implant was scattered rather than spherical. In 9/20 (45%), the implants were not located in the intended mid-urethral position. In 8/20 patients (40%), the material was distributed circumferentially.
Conclusion: This is the first study describing the position and shape of VDPDMS in patients after treatment. The appearance and position of the implants appears to be variable, but optimal positioning or shape seems to be no absolute requisite for success.
Keywords: computed tomography; stress urinary incontinence; urethral bulking agent; urolastic.
© The Author(s), 2021.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.M.d.V. reports non-financial support from Astellas Pharma BV, grants from Urogyn BV, during the conduct of the study; and family ties with former shareholders in Urogyn BV, Nijmegen, The Netherlands. J.P.W.R.R. reports consultancy at Coloplast, consultancy at Promedon, outside the submitted work; J.P.F.A.H. reports grants from Urogyn BV, during the conduct of the study; grants and personal fees from Astellas, grants from Bluewind, personal fees from Pierre Fabre, personal fees from Ixaltis, outside the submitted work. F.M.C. and J.J.F. have no conflict of interest to disclose.
Figures



Similar articles
-
Results of an innovative bulking agent in patients with stress urinary incontinence who are not optimal candidates for mid-urethral sling surgery.Neurourol Urodyn. 2018 Jan;37(1):339-345. doi: 10.1002/nau.23299. Epub 2017 Apr 28. Neurourol Urodyn. 2018. PMID: 28452427
-
Para-Urethral Injections with Urolastic® for Treatment of Female Stress Urinary Incontinence: Subjective Improvement and Safety.Urol Int. 2017;99(1):91-97. doi: 10.1159/000452450. Epub 2017 Feb 3. Urol Int. 2017. PMID: 28152525 Free PMC article.
-
An Open Multicenter Study of Clinical Efficacy and Safety of Urolastic, an Injectable Implant for the Treatment of Stress Urinary Incontinence: One-Year Observation.Biomed Res Int. 2015;2015:851823. doi: 10.1155/2015/851823. Epub 2015 Apr 20. Biomed Res Int. 2015. PMID: 26106616 Free PMC article.
-
Urolastic®, a new bulking agent for treatment of stress urinary incontinence: a systematic review and meta-analysis.Int Urogynecol J. 2018 Sep;29(9):1239-1247. doi: 10.1007/s00192-018-3703-6. Epub 2018 Jun 23. Int Urogynecol J. 2018. PMID: 29934769
-
Injectable biomaterials for the treatment of stress urinary incontinence: their potential and pitfalls as urethral bulking agents.Int Urogynecol J. 2013 Jun;24(6):913-9. doi: 10.1007/s00192-012-2011-9. Epub 2012 Dec 8. Int Urogynecol J. 2013. PMID: 23224022 Review.
Cited by
-
Current Treatment of Stress Urinary Incontinence by Bulking Agents and Laser Therapy-An Update.J Clin Med. 2024 Feb 28;13(5):1377. doi: 10.3390/jcm13051377. J Clin Med. 2024. PMID: 38592248 Free PMC article. Review.
References
-
- Burkhard FC, Bosch J, Cruz F, et al.. EAU-guidelines on urinary incontinence in adults 2018, https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Urinary-Incontin...
-
- Chapple CR, Cruz F, Deffieux X, et al.. Consensus statement of the European Urology Association and the European Urogynaecological Association on the use of implanted materials for treating pelvic organ prolapse and stress urinary incontinence. Eur Urol 2017; 72: 424–431. - PubMed
-
- de Vries AM, Venema PL, Heesakkers JPFA. Midurethral support is also necessary for reflex closure of the urethra. Neurourol Urodyn 2018; 37: 2965–2972. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

