Cabazitaxel suppresses the proliferation and promotes the apoptosis and radiosensitivity of castration-resistant prostate cancer cells by inhibiting PI3K/AKT pathway
- PMID: 35173836
- PMCID: PMC8829643
Cabazitaxel suppresses the proliferation and promotes the apoptosis and radiosensitivity of castration-resistant prostate cancer cells by inhibiting PI3K/AKT pathway
Abstract
Background: Cabazitaxel has been applied to the treatment of castration-resistant prostate cancer (CRPC), but the molecular mechanism remained to be fully understood.
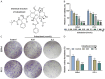
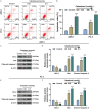
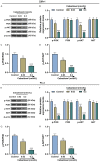
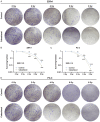
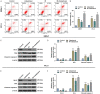
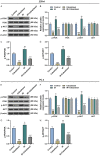
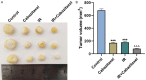
Methods: After treatment with Cabazitaxel alone or in combination with ionizing radiation (IR), CRPC cell viability, proliferation and apoptosis were determined by Cell Counting Kit-8 (CCK-8) assay, colony formation, and flow cytometry, respectively. Tumor volume was measured after the establishment of animal xenograft model. Relative expressions of proteins related to apoptosis (B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), and cleaved caspase 3) and phosphoinositide 3-kinase (PI3K)/AKT pathway were measured by Western blot, and the phosphorylated-PI3K/PI3K and p-AKT/AKT ratios were determined as well.
Results: Cell viability and proliferation were suppressed, and apoptosis was promoted in CRPC cells after Cabazitaxel treatment alone, accompanied with upregulated expressions of Bax and cleaved caspase 3 and downregulated Bcl-2 expression. Also, a single treatment with Cabazitaxel resulted in suppression of PI3K/AKT pathway activation, along with downregulated expressions of p-PI3K and p-AKT and a reduced ratio of p-PI3K/PI3K to p-AKT/AKT. Meanwhile, Cabazitaxel enhanced the effects of IR on suppressing survival and promoting apoptosis in CRPC cells through downregulating Bcl-2 and upregulating Bax and cleaved caspase 3. However, Cabazitaxel suppressed IR-induced PI3K/AKT pathway activation via downregulating p-PI3K and p-AKT, leading to a reduced ratio of p-PI3K/PI3K to p-AKT/AKT. Furthermore, Cabazitaxel further promoted the effects of IR on suppressing tumor growth in vivo.
Conclusion: Cabazitaxel inhibited the proliferation and promoted the apoptosis and radiosensitivity of CRPC cells, which is related to the suppression of PI3K/AKT pathway, providing a therapeutic method for CRPC in clinical practice.
Keywords: Castration resistant prostate cancer; apoptosis; cabazitaxel; phosphoinositide 3-kinase (PI3K)/AKT pathway; proliferation; radiosensitivity.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures
Similar articles
-
Lobaplatin induces apoptosis in T24 and 5637 bladder cancer cells by regulating Bcl-2 and Bax expression and inhibiting the PI3K/Akt signaling pathway.Transl Androl Urol. 2023 Aug 31;12(8):1296-1307. doi: 10.21037/tau-23-376. Epub 2023 Aug 28. Transl Androl Urol. 2023. PMID: 37680227 Free PMC article.
-
Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer.Cancer Sci. 2018 Sep;109(9):2937-2945. doi: 10.1111/cas.13729. Epub 2018 Aug 6. Cancer Sci. 2018. PMID: 29989268 Free PMC article.
-
Rooibos suppresses proliferation of castration-resistant prostate cancer cells via inhibition of Akt signaling.Phytomedicine. 2019 Nov;64:153068. doi: 10.1016/j.phymed.2019.153068. Epub 2019 Aug 8. Phytomedicine. 2019. PMID: 31419729
-
Pyrogallol from Spirogyra neglecta Inhibits Proliferation and Promotes Apoptosis in Castration-Resistant Prostate Cancer Cells via Modulating Akt/GSK-3β/β-catenin Signaling Pathway.Int J Mol Sci. 2023 Mar 29;24(7):6452. doi: 10.3390/ijms24076452. Int J Mol Sci. 2023. PMID: 37047425 Free PMC article.
-
Effects of Glut1 gene silencing on proliferation, differentiation, and apoptosis of colorectal cancer cells by targeting the TGF-β/PI3K-AKT-mTOR signaling pathway.J Cell Biochem. 2018 Feb;119(2):2356-2367. doi: 10.1002/jcb.26399. Epub 2017 Oct 18. J Cell Biochem. 2018. Retraction in: J Cell Biochem. 2021 Nov;122 Suppl 1:S101. doi: 10.1002/jcb.30081. PMID: 28884839 Retracted.
Cited by
-
Future Treatment Strategies for Cancer Patients Combining Targeted Alpha Therapy with Pillars of Cancer Treatment: External Beam Radiation Therapy, Checkpoint Inhibition Immunotherapy, Cytostatic Chemotherapy, and Brachytherapy.Pharmaceuticals (Basel). 2024 Aug 5;17(8):1031. doi: 10.3390/ph17081031. Pharmaceuticals (Basel). 2024. PMID: 39204136 Free PMC article. Review.
-
Targeting PI3K/Akt signaling in prostate cancer therapy.J Cell Commun Signal. 2023 Sep;17(3):423-443. doi: 10.1007/s12079-022-00702-1. Epub 2022 Nov 11. J Cell Commun Signal. 2023. PMID: 36367667 Free PMC article. Review.
-
Important Roles of PI3K/AKT Signaling Pathway and Relevant Inhibitors in Prostate Cancer Progression.Cancer Med. 2024 Nov;13(21):e70354. doi: 10.1002/cam4.70354. Cancer Med. 2024. PMID: 39485722 Free PMC article. Review.
-
BMI1 governs the maintenance of mouse GC-2 cells through epigenetic repression of Foxl1 transcription.Am J Transl Res. 2022 May 15;14(5):3407-3418. eCollection 2022. Am J Transl Res. 2022. PMID: 35702123 Free PMC article.
-
Synergistic Anti-tumorigenic Effects of Cabazitaxel and Usnic Acid Combination on Metastatic Castration-Resistant Prostate Cancer Cells.Anticancer Agents Med Chem. 2025;25(9):610-619. doi: 10.2174/0118715206336754241015062614. Anticancer Agents Med Chem. 2025. PMID: 39810522
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Bonfill X, Arevalo-Rodriguez I, Martinez Garcia L, Quintana MJ, Buitrago-Garcia D, Lobos Urbina D, Cordero JA. Intermittent androgen deprivation therapy: recommendations to improve the management of patients with prostate cancer following the GRADE approach. Cancer Manag Res. 2018;10:2357–2367. - PMC - PubMed
-
- Cao Q, Song Z, Ruan H, Wang C, Yang X, Bao L, Wang K, Cheng G, Xu T, Xiao W, Xiong Z, Liu D, Yang M, Zhou D, Yang H, Chen K, Zhang X. Targeting the KIF4A/AR axis to reverse endocrine therapy resistance in castration-resistant prostate cancer. Clin Cancer Res. 2020;26:1516–1528. - PubMed
-
- Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, Kopka K, Apostolidis C, Haberkorn U, Morgenstern A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
