Clinical efficacy of Weisu granule combined with Weifuchun tablet in the treatment of chronic atrophic gastritis and its effect on serum G-17, PG I and PG II levels
- PMID: 35173844
- PMCID: PMC8829644
Clinical efficacy of Weisu granule combined with Weifuchun tablet in the treatment of chronic atrophic gastritis and its effect on serum G-17, PG I and PG II levels
Abstract
Objective: The aim of this prospective study was to explore the clinical efficacy of Weisu granules combined with Weifuchun tablets in the treatment of chronic atrophic gastritis and its effect on serum gastrin-17 (G-17), pepsinogen I (PG I), and II (PG II) levels.
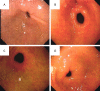
Methods: Totally, 120 patients with chronic atrophic gastritis admitted to our hospital from February 2019 to February 2020 were enrolled and randomized into a control group (n=60) treated with Weifuchun tablets, and a experimental group given Weisu granules. Serum G-17, PG I, and PG II levels, inflammatory factor levels, TCM syndrome scores, gastric mucosa pathological scores, and clinical efficacy were compared between the two groups. Gastric tissue changes were observed using gastroscopy and HE staining.
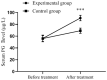
Results: After treatment, the levels of serum G-17, PG I, and PG II of the experimental group were significantly better than those of the control group (P<0.001). The levels of inflammatory factors, traditional Chinese medicine (TCM) syndrome scores, and gastric mucosal pathology scores of the experimental group were significantly lower than those of the control group (P<0.001). The overall response rate of the experimental group was significantly higher than that of the control group (P<0.05). The experimental group showed a lower HP positive result and a higher HP negative conversion ratio than the control group (all P<0.05). HE staining results revealed that after treatment, the number of glands was basically restored to the level of normal gastric mucosa, and the improvement of inflammatory cell infiltration in the experimental group was significantly better than that in the control group.
Conclusion: Weisu granule combined with Weifuchun tablets can ameliorate serum G-17, PG I, and PG II levels in patients with chronic atrophic gastritis, relieve inflammatory responses and clinical symptoms, and improve the treatment effect, which is worth promoting in clinical practice.
Clinical trial registration: Chinese Registry of Clinical Trials.
Trial registration number: ChiCTR200002548416. Trial URL: http://www.chictr.org.cn/showproj.aspx?proj=26516901.
Keywords: Weifuchun tablets; Weisu granules; chronic atrophic gastritis.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures



Similar articles
-
Efficacy and safety of Weifuchun tablet for chronic atrophic gastritis: A systematic review and meta-analysis.PLoS One. 2023 Apr 13;18(4):e0284411. doi: 10.1371/journal.pone.0284411. eCollection 2023. PLoS One. 2023. PMID: 37053262 Free PMC article.
-
[Clinical effects of acupoint catgut embedding with quadruple therapy on Hp (+) chronic atro-phic gastritis of spleen and stomach deficiency syndrome].Zhen Ci Yan Jiu. 2022 Jun 25;47(6):537-43. doi: 10.13702/j.1000-0607.20210502. Zhen Ci Yan Jiu. 2022. PMID: 35764522 Chinese.
-
Chinese herbal compound prescriptions combined with Chinese medicine powder based on traditional Chinese medicine syndrome differentiation for treatment of chronic atrophic gastritis with erosion: a multi-center, randomized, positive-controlled clinical trial.Chin Med. 2022 Dec 22;17(1):142. doi: 10.1186/s13020-022-00692-7. Chin Med. 2022. PMID: 36550503 Free PMC article.
-
Yiwei Xiaoyu granules for treatment of chronic atrophic gastritis with deficiency syndrome of the spleen and stomach.World J Clin Cases. 2024 May 6;12(13):2201-2209. doi: 10.12998/wjcc.v12.i13.2201. World J Clin Cases. 2024. PMID: 38808353 Free PMC article. Clinical Trial.
-
[Systematic review and Meta-analysis of efficacy and safety of Yangwei Granules for chronic gastritis].Zhongguo Zhong Yao Za Zhi. 2020 Oct;45(20):5008-5016. doi: 10.19540/j.cnki.cjcmm.20200314.503. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 33350276 Chinese.
Cited by
-
Progress in traditional Chinese medicine against chronic gastritis: From chronic non-atrophic gastritis to gastric precancerous lesions.Heliyon. 2023 May 27;9(6):e16764. doi: 10.1016/j.heliyon.2023.e16764. eCollection 2023 Jun. Heliyon. 2023. PMID: 37313135 Free PMC article. Review.
-
New opportunities and challenges of natural products research: When target identification meets single-cell multiomics.Acta Pharm Sin B. 2022 Nov;12(11):4011-4039. doi: 10.1016/j.apsb.2022.08.022. Epub 2022 Aug 27. Acta Pharm Sin B. 2022. PMID: 36386472 Free PMC article. Review.
-
Therapeutic Potential of Qilianxiaopi Formula: Targeting ADAM17-Mediated Chronic Inflammation in Atrophic Gastritis.Pharmaceuticals (Basel). 2025 Mar 19;18(3):435. doi: 10.3390/ph18030435. Pharmaceuticals (Basel). 2025. PMID: 40143211 Free PMC article.
-
Characteristics of different types of Helicobacter pylori: New evidence from non-amplified white light endoscopy.Front Microbiol. 2023 Jan 13;13:999564. doi: 10.3389/fmicb.2022.999564. eCollection 2022. Front Microbiol. 2023. PMID: 36713187 Free PMC article.
-
Efficacy and safety of Weifuchun tablet for chronic atrophic gastritis: A systematic review and meta-analysis.PLoS One. 2023 Apr 13;18(4):e0284411. doi: 10.1371/journal.pone.0284411. eCollection 2023. PLoS One. 2023. PMID: 37053262 Free PMC article.
References
-
- Holleczek B, Schttker B, Brenner H. Helicobacter pylori infection, chronic atrophic gastritis and risk of stomach and esophagus cancer: results from the prospective population-based ESTHER cohort study. Int J Cancer. 2020;146:2773–2783. - PubMed
-
- Tian G, Wu C, Li J, Liang B, Zhang F, Fan X, Li Z, Wang Y, Li Z, Liu D, Lai-Han Leung E, Chen J. Network pharmacology based investigation into the effect and mechanism of modified sijunzi decoction against the subtypes of chronic atrophic gastritis. Pharmacol Res. 2019;144:158–166. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
