Repair phase modeling of ischemic acute kidney injury: recovery vs. transition to chronic kidney disease
- PMID: 35173874
- PMCID: PMC8829619
Repair phase modeling of ischemic acute kidney injury: recovery vs. transition to chronic kidney disease
Abstract
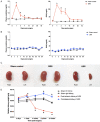
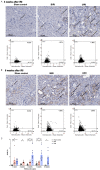
The repair mechanism after ischemic acute kidney injury (AKI) involves complex immunologic processes, which determine long-term renal outcomes. Through investigating two murine ischemia-reperfusion injury (IRI) models: bilateral IRI (BIRI) and unilateral IRI (UIRI), we aimed to determine an appropriate murine model that could simulate the recovery phase of ischemic AKI. Changes in renal function, phenotypes of kidney mononuclear cells, renal fibrosis, and intrarenal cytokine/chemokine expression were serially analyzed up to 12 weeks after IRI. Plasma creatinine and BUN concentrations increased and remained elevated in the BIRI group until 7 days but decreased to comparable levels with the sham control group at 2 weeks after surgery and thereafter, whereas plasma creatinine and BUN concentrations remained unchanged in the UIRI group. Intrarenal total leukocytes, and effector memory and activated phenotypes of CD4 and CD8 T cells markedly increased in the postischemic kidneys in both IRI groups. Expression of proinflammatory cytokines/chemokines and TGF-β1 was enhanced in the postischemic kidneys of both IRI groups with a higher degree in the UIRI group. Importantly, intrarenal immunologic changes of the BIRI group persisted until 6 weeks despite full functional recovery. The postischemic kidneys of the UIRI group showed earlier and more pronounced proinflammatory conditions as well as more severe atrophic and fibrotic changes compared to the BIRI group. These findings support the utility of longer follow-ups of BIRI and UIRI models for investigating the adaptive repair process, which facilitates recovery of ischemic AKI and maladaptive repair process may result in AKI to CKD transition, respectively.
Keywords: Ischemic acute kidney injury; chronic kidney disease; ischemia-reperfusion injury; recovery; repair.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures






Similar articles
-
Effects of contralateral nephrectomy timing and ischemic conditions on kidney fibrosis after unilateral kidney ischemia-reperfusion injury.Ren Fail. 2022 Dec;44(1):1568-1584. doi: 10.1080/0886022X.2022.2126790. Ren Fail. 2022. PMID: 36154902 Free PMC article.
-
Dietary modifications affect renal recovery during the healing phase following ischemic acute ischemic kidney injury.Front Cell Dev Biol. 2025 May 22;13:1494660. doi: 10.3389/fcell.2025.1494660. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40476004 Free PMC article.
-
Aging has small effects on initial ischemic acute kidney injury development despite changing intrarenal immunologic micromilieu in mice.Am J Physiol Renal Physiol. 2016 Feb 15;310(4):F272-83. doi: 10.1152/ajprenal.00217.2015. Epub 2015 Dec 9. Am J Physiol Renal Physiol. 2016. PMID: 26661651
-
Metabolic Flexibility and Innate Immunity in Renal Ischemia Reperfusion Injury: The Fine Balance Between Adaptive Repair and Tissue Degeneration.Front Immunol. 2020 Jul 7;11:1346. doi: 10.3389/fimmu.2020.01346. eCollection 2020. Front Immunol. 2020. PMID: 32733450 Free PMC article. Review.
-
Immune Cells in Ischemic Acute Kidney Injury.Curr Protein Pept Sci. 2019;20(8):770-776. doi: 10.2174/1389203720666190507102529. Curr Protein Pept Sci. 2019. PMID: 31060484 Review.
Cited by
-
Ferroptosis in Cancer and Inflammatory Diseases: Mechanisms and Therapeutic Implications.MedComm (2020). 2025 Sep 3;6(9):e70349. doi: 10.1002/mco2.70349. eCollection 2025 Sep. MedComm (2020). 2025. PMID: 40919133 Free PMC article. Review.
-
Energy metabolic reprogramming regulates programmed cell death of renal tubular epithelial cells and might serve as a new therapeutic target for acute kidney injury.Front Cell Dev Biol. 2023 Nov 20;11:1276217. doi: 10.3389/fcell.2023.1276217. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38054182 Free PMC article. Review.
References
-
- Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. - PMC - PubMed
-
- Kurzhagen JT, Dellepiane S, Cantaluppi V, Rabb H. AKI: an increasingly recognized risk factor for CKD development and progression. J Nephrol. 2020;33:1171–1187. - PubMed
-
- Kumar S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 2018;93:27–40. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
