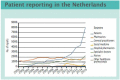
The value of direct patient reporting in pharmacovigilance
- PMID: 35173952
- PMCID: PMC8842177
- DOI: 10.1177/2042098620940164
The value of direct patient reporting in pharmacovigilance
Keywords: direct patient reporting; healthcare; patients; pharmacovigilance.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Sten Olsson. President of ISoP and former manager of the UMC international pharmacovigilance training courses and editor of Uppsala Reports, 2 October 2017.
-
- Cameron M, Miller AR, Chacko B, et al.. FDA reported use of patient experience data in 2018 drug approvals. Ther Innov Regul Sci 2020; 54: 709–716. - PubMed
-
- Van Hulsen F, Harmark L, Rolfes L. 15 years of patient reporting: what have we learnt and where are we heading to? Expert Opin Drug Saf 2019; 18: 477–484. - PubMed
-
- Scholl J. Geneesmiddelenbewaking door Lareb. Verleden, heden en toekomst. Bijwerkingen centrum Lareb, 2015. https://adoc.pub/geneesmiddelenbewaking-door-lareb.html
-
- European Medicines Agency. QPPV Update, August 2019.
Publication types
LinkOut - more resources
Full Text Sources