Updated consensus statements on COVID-19 Vaccine Allergy Safety in Hong Kong
- PMID: 35174059
- PMCID: PMC8819424
- DOI: 10.5415/apallergy.2022.12.e8
Updated consensus statements on COVID-19 Vaccine Allergy Safety in Hong Kong
Abstract
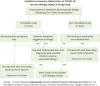
Due to global concerns over coronavirus disease 2019 (COVID-19) vaccine-associated allergic reactions; the Hong Kong Institute of Allergy (HKIA) formulated an initial set of consensus statements (CS) on COVID-19 Vaccine Allergy Safety (VAS) in early 2021. Following accumulation of both local and international experience on and COVID-19 VAS, the HKIA task force reformed to update the Hong Kong consensus on COVID-19 VAS. A nominated task force of experts managing patients with drug and vaccine allergies in Hong Kong formulated the updated CS by unanimous decision. A total of 9 new statements were established. Individuals with history of food allergies and anaphylaxis unrelated to the components of COVID-19 vaccines do not require allergist review prior to vaccination. Individuals with history suspicious of an excipient allergy may now be vaccinated with a non-PEG containing vaccine without prior allergist assessment. Individuals with suspected mild allergic reactions following prior COVID-19 vaccination can proceed with the next dose. Only individuals who present with immediate-type allergic reaction with systemic symptoms or more severe nonimmediate type reactions should defer their next dose until allergist review. The remaining statements regarding adequate safety during vaccination and advocation for legislative changes regarding excipient disclosure in Hong Kong remained unchanged from the prior CS. The updated CS are updated in accordance with local and international experience thus far and serve as guidance for local frontline healthcare providers to further promote safe COVID-19 vaccine uptake in Hong Kong.
Keywords: Allergy; COVID-19; Vaccine; Vaccine allergy.
Copyright © 2022. Asia Pacific Association of Allergy, Asthma and Clinical Immunology.
Conflict of interest statement
Conflict of Interest: The authors have no financial conflicts of interest.
Figures
References
-
- Hong Kong Vaccination Dashboard [Internet] Hong Kong: The government of the Hong Kong Special Administrative Region; 2021. [cited 2021 Jul 21]. Available from: https://www.covidvaccine.gov.hk/en/
Publication types
LinkOut - more resources
Full Text Sources