Imaging Biomarkers of Glioblastoma Treatment Response: A Systematic Review and Meta-Analysis of Recent Machine Learning Studies
- PMID: 35174084
- PMCID: PMC8842649
- DOI: 10.3389/fonc.2022.799662
Imaging Biomarkers of Glioblastoma Treatment Response: A Systematic Review and Meta-Analysis of Recent Machine Learning Studies
Erratum in
-
Erratum: Imaging biomarkers of glioblastoma treatment response: a systematic review and meta-analysis of recent machine learning studies.Front Oncol. 2023 May 24;13:1217461. doi: 10.3389/fonc.2023.1217461. eCollection 2023. Front Oncol. 2023. PMID: 37293592 Free PMC article.
Abstract
Objective: Monitoring biomarkers using machine learning (ML) may determine glioblastoma treatment response. We systematically reviewed quality and performance accuracy of recently published studies.
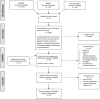
Methods: Following Preferred Reporting Items for Systematic Reviews and Meta-Analysis: Diagnostic Test Accuracy, we extracted articles from MEDLINE, EMBASE and Cochrane Register between 09/2018-01/2021. Included study participants were adults with glioblastoma having undergone standard treatment (maximal resection, radiotherapy with concomitant and adjuvant temozolomide), and follow-up imaging to determine treatment response status (specifically, distinguishing progression/recurrence from progression/recurrence mimics, the target condition). Using Quality Assessment of Diagnostic Accuracy Studies Two/Checklist for Artificial Intelligence in Medical Imaging, we assessed bias risk and applicability concerns. We determined test set performance accuracy (sensitivity, specificity, precision, F1-score, balanced accuracy). We used a bivariate random-effect model to determine pooled sensitivity, specificity, area-under the receiver operator characteristic curve (ROC-AUC). Pooled measures of balanced accuracy, positive/negative likelihood ratios (PLR/NLR) and diagnostic odds ratio (DOR) were calculated. PROSPERO registered (CRD42021261965).
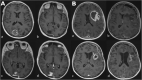
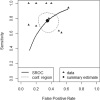
Results: Eighteen studies were included (1335/384 patients for training/testing respectively). Small patient numbers, high bias risk, applicability concerns (particularly confounding in reference standard and patient selection) and low level of evidence, allow limited conclusions from studies. Ten studies (10/18, 56%) included in meta-analysis gave 0.769 (0.649-0.858) sensitivity [pooled (95% CI)]; 0.648 (0.749-0.532) specificity; 0.706 (0.623-0.779) balanced accuracy; 2.220 (1.560-3.140) PLR; 0.366 (0.213-0.572) NLR; 6.670 (2.800-13.500) DOR; 0.765 ROC-AUC.
Conclusion: ML models using MRI features to distinguish between progression and mimics appear to demonstrate good diagnostic performance. However, study quality and design require improvement.
Keywords: artificial intelligence; deep learning; glioblastoma; glioma; machine learning; meta-analysis; monitoring biomarkers; treatment response.
Copyright © 2022 Booth, Grzeda, Chelliah, Roman, Al Busaidi, Dragos, Shuaib, Luis, Mirchandani, Alparslan, Mansoor, Lavrador, Vergani, Ashkan, Modat and Ourselin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and Other Tools) Resource. 1st edn. Silver Spring, MD: Food and Drug Administration (US), co-published by Bethesda, MD: National Institutes of Health US; (2016). Available at: https://www.ncbi.nlm.nih.gov/books/NBK326791. - PubMed
-
- Booth TC, Tang Y, Waldman AD, Quigley A-M, Lewis D, Soloviev D, et al. Neuro-Oncology Single-Photon Emission CT: A Current Overview. Neurographics (2011) 01:108–20. doi: 10.3174/ng.3110014 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

