Mucus Hypersecretion and Ciliary Impairment in Conducting Airway Contribute to Alveolar Mucus Plugging in Idiopathic Pulmonary Fibrosis
- PMID: 35174169
- PMCID: PMC8842394
- DOI: 10.3389/fcell.2021.810842
Mucus Hypersecretion and Ciliary Impairment in Conducting Airway Contribute to Alveolar Mucus Plugging in Idiopathic Pulmonary Fibrosis
Abstract
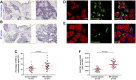
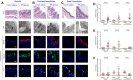
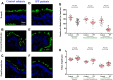
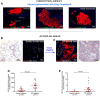
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease attributed to the complex interplay of genetic and environmental risks. The muco-ciliary clearance (MCC) system plays a critical role in maintaining the conduit for air to and from the alveoli, but it remains poorly understood whether the MCC abnormalities in conducting airway are involved in IPF pathogenesis. In this study, we obtained the surgically resected bronchi and peripheral lung tissues from 31 IPF patients and 39 control subjects, and we sought to explore the morphologic characteristics of MCC in conducting airway by using immunostaining and scanning and transmission electron microscopy. In the submucosal regions of the bronchi, we found that the areas of mucus glands (MUC5B+) were significantly larger in IPF patients as compared with control subjects (p < 0.05). In the surface epithelium of three airway regions (bronchi, proximal bronchioles, and distal bronchioles), increased MUC5B and MUC5AC expression of secretory cells, decreased number of ciliated cells, and increased ciliary length were observed in IPF patients than control subjects (all p < 0.05). In addition, the mRNA expression levels of MUC5B were up-regulated in both the bronchi and peripheral lung of IPF patients than those of control subjects (p < 0.05), accompanied with 93.55% IPF subjects who had obvious MUC5B+ mucus plugs in alveolar regions. No MUC5B rs35705950 single-nucleotide polymorphism allele was detected in both IPF patients and control subjects. Our study shows that mucus hypersecretion and ciliary impairment in conducting airway are major causes of mucus plugs in alveolar regions and may be closely related to the alveolar injuries in IPF patients.
Keywords: MUC5B; conducting airway mucosa; idiopathic pulmonary fibrosis; muco-ciliary clearance; mucus plugs.
Copyright © 2022 Peng, Wang, Xu, Fang, Chen, Hou, Zhou, Lin, Xie, Tang, Wang and Zhong.
Conflict of interest statement
Author X-TH is employed by Guangzhou KingMed Center for Clinical Laboratory Co., Ltd., Guangzhou, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Allen R. J., Porte J., Braybrooke R., Flores C., Fingerlin T. E., Oldham J. M., et al. (2017). Genetic Variants Associated With Susceptibility to Idiopathic Pulmonary Fibrosis in People of European Ancestry: A Genome-Wide Association Study. Lancet Respir. Med. 5 (11), 869–880. 10.1016/S2213-2600(17)30387-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

