Neutrophil-Platelet Interactions as Novel Treatment Targets in Cardiovascular Disease
- PMID: 35174225
- PMCID: PMC8841491
- DOI: 10.3389/fcvm.2021.824112
Neutrophil-Platelet Interactions as Novel Treatment Targets in Cardiovascular Disease
Abstract
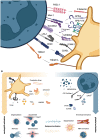
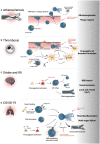
Neutrophils and platelets are among the most abundant cell types in peripheral blood and characterized by high plasticity and a readily available reservoir of surface proteins and secretable granule contents. Receptor-mediated activation and granule release predispose both cell types for rapid responses to various stimuli. While neutrophils provide the first line of defense to microbial infections and platelets are known for their aggregatory functions in hemostasis and thrombosis, research of the past decade has highlighted that both cell types jointly shape local and systemic immune responses and clot formation alike. Concomitant activation of neutrophils and platelets has been observed in a variety of cardiovascular diseases, including arterial and venous thrombosis, atherosclerosis as well as myocardial infarction and ischemia-reperfusion injury. In this review, we describe the mechanisms by which neutrophils and platelets interact physically, how release of granule contents and soluble molecules by either cell type affects the other and how this mutual activation supports the efficacy of immune responses. We go on to describe how activated platelets contribute to host defense by triggering neutrophil extracellular trap (NET) formation in a process termed immunothrombosis, which in turn promotes local platelet activation and coagulation. Further, we review current evidence of hazardous overactivation of either cell type and their respective role in cardiovascular disease, with a focus on thrombosis, myocardial infarction and ischemia-reperfusion injury, and describe how neutrophils and platelets shape thromboinflammation in COVID-19. Finally, we provide an overview of therapeutic approaches targeting neutrophil-platelet interactions as novel treatment strategy in cardiovascular disease.
Keywords: NETosis; cardiovascular disease; neutrophil; platelet; thrombosis.
Copyright © 2022 Kaiser, Escaig, Erber and Nicolai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Silvis MJM, Kaffka Genaamd Dengler SE, Odille CA, Mishra M, van der Kaaij NP, Doevendans PA, et al. . Damage-Associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Front Immunol. (2020) 11:599511. 10.3389/fimmu.2020.599511 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

