A wearable cardioverter defibrillator with a low false alarm rate
- PMID: 35174572
- PMCID: PMC9305432
- DOI: 10.1111/jce.15417
A wearable cardioverter defibrillator with a low false alarm rate
Abstract
Introduction: A wearable cardioverter defibrillator (WCD) is indicated in appropriate patients to reduce the risk for sudden cardiac death. Challenges for patients wearing a WCD have been frequent false shock alarms primarily due to electrocardiogram noise and wear discomfort. The objective of this study was to test a contemporary WCD designed for reduced false shock alarms and improved comfort.
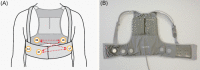
Methods: One hundred and thirty patients with left ventricular ejection fraction ≤40% and an active implantable cardioverter defibrillator (ICD) were fitted with the ASSURE WCD (Kestra Medical Technologies) and followed for 30 days. WCD detection was enabled and shock alarm markers recorded, but shocks and shock alarms were disabled. All WCD episodes and ICD ventricular tachycardia/ventricular fibrillation (VT/VF) episodes were adjudicated. The primary endpoint was the false-positive shock alarm rate with a performance goal of 1 every 3.4 days (0.29 per patient-day).
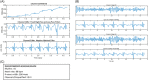
Results: Of 163 WCD episodes, 4 were VT/VF and 159 non-VT/VF (121 rhythms with noise, 32 uncertain with noise, 6 atrial flutter without noise). Only three false-positive shock alarm markers were recorded; one false-positive shock alarm every 1333 patient-days (0.00075 per patient-day, 95% confidence interval: 0.00015-0.00361; p < .001). No ICD recorded VT/VF episodes meeting WCD detection criteria (≥170 bpm for ≥20 s) were missed by the WCD during 3501 patient-days of use. Median wear was 31.0 days (interquartile range [IQR] 2.0) and median daily use 23.0 h (IQR 1.7). Adverse events were mostly mild: skin irritation (19.4%) and musculoskeletal discomfort (8.5%).
Conclusion: The ASSURE WCD demonstrated a low false-positive shock alarm rate, low patient-reported discomfort, and no serious adverse events.
Keywords: defibrillator; sudden cardiac death; ventricular arrhythmia.
© 2022 The Authors. Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Figures


Comment in
-
The wearable cardioverter-defibrillator-Improving comfort and reaching towards noise immunity.J Cardiovasc Electrophysiol. 2022 May;33(5):843-844. doi: 10.1111/jce.15416. Epub 2022 Feb 27. J Cardiovasc Electrophysiol. 2022. PMID: 35175650 Free PMC article. No abstract available.
References
-
- Piccini JP, Sr, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP. American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Wearable cardioverter‐defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American Heart Association. Circulation. 2016;133(17):1715‐1727. 10.1161/CIR.0000000000000394 - DOI - PubMed
-
- Al‐Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e190‐e252. 10.1016/j.hrthm.2017.10.035 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

