Acquired haemophilia A: Italian Consensus Recommendations on diagnosis, general management and treatment of bleeding
- PMID: 35175184
- PMCID: PMC9068356
- DOI: 10.2450/2022.0238-21
Acquired haemophilia A: Italian Consensus Recommendations on diagnosis, general management and treatment of bleeding
Abstract
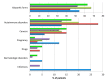
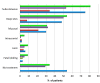
Background: Acquired haemophilia A (AHA) is a rare bleeding disorder due to autoantibodies to coagulation factor VIII that may be secondary to autoimmune diseases, cancer, drugs, pregnancy, infections, or be idiopathic. Recurrent bleeding, often severe, mostly in muscles and soft tissues, and isolated prolonged activated partial thromboplastin time (aPTT), in the absence of personal and family history of bleeding, are typical features that should raise the suspicion of AHA. Poor awareness of the disease results in diagnostic delays and inappropriate treatment.
Materials and methods: The Italian Association of Haemophilia Centres (AICE) developed consensus recommendations in cooperation with the Italian Society on Thrombosis and Haemostasis (SISET). The document was shared with scientific societies of specialist physicians, laboratory professionals and pharmacists to spread knowledge about AHA and promote appropriate diagnosis/treatment.
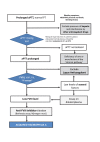
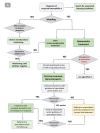
Results: Ready availability of the aPTT mixing test is crucial, although diagnostic confirmation and optimal management require prompt referral of patients to specialised centres with rapidly available diagnostic and therapeutic facilities. If immediate referral is unfeasible, treatment must be undertaken early, under guidance of specialised centres or based on shared protocols. Recommendations about diagnosis, general management and, in bleeding patients, haemostatic therapy using bypassing agents or replacement treatment, including the recently available recombinant porcine factor VIII, are provided, considering the different clinical settings and laboratory facilities.
Discussion: This consensus document aims to improve the overall healthcare pathways for AHA, harmonise the management and therapeutic approaches to newly diagnosed patients and reduce the still relevant complications and mortality in this setting.
Conflict of interest statement
AC has acted as a paid consultant or speaker for Bayer, Kedrion, Novo Nordisk, Roche and Werfen. MF has acted as a paid consultant or speaker for Bayer and Novo Nordisk. AT has received speaker fees from Roche, Stago, Sobi and Werfen. GC has participated as a speaker or in advisory boards for Alexion, CSL Behring, Kedrion, Novo Nordisk, Pfizer, Sanofi, Sobi, Takeda, Uniqure and Werfen, acted as a consultant to Roche, and received research funding directly to his Institution from CSL Behring, Pfizer and Sobi. CS has acted as a paid consultant/advisor/speaker for Amgen, Bayer, Biomarin, CSL Behring, Novo Nordisk, Roche, Sobi and Takeda. LC has received speaker fees from Bayer and Novo Nordisk. RDC has received speaker fees from Bayer, Sanofi, Roche, Takeda, and Sobi and participated in scientific advisory boards for Bayer, Sanofi, Pfizer, Werfen, Sobi and Kedrion. ACM has acted as an advisor for Bayer, CSL Behring, Roche, SOBI and Takeda and has received speaker fees from Bayer, CSL Behring, Kedrion, Novo Nordisk, Pfizer, Roche, SOBI and Takeda. PG has received speaker fees from Sanofi. AR has acted as a paid consultant/advisor/speaker for Bayer, CSL Behring, Shire/Takeda, Novo Nordisk, Kedrion, Pfizer, Roche and Sobi. RCS, RM, EZ and GFR declare that they have no interests which might be perceived as conflicts or bias.
Figures

References
-
- Franchini M, Vaglio S, Marano G, et al. Acquired hemophilia A: a review of recent data and new therapeutic options. Hematology. 2017;25:1–7. - PubMed
-
- Coppola A, Favaloro EJ, Tufano A, et al. Acquired inhibitors of coagulation factors: part I - acquired hemophilia A. Semin Thromb Hemost. 2012;38:433–46. - PubMed
-
- Collins PW, Hirsch S, Baglin TP, et al. for the UK Haemophilia Centre Doctors’ Organisation. Acquired haemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109:1870–7. - PubMed
-
- Knoebl P, Marco P, Baudo F, et al. on behalf of the EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2) J Thromb Haemost. 2012;10:622–31. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
